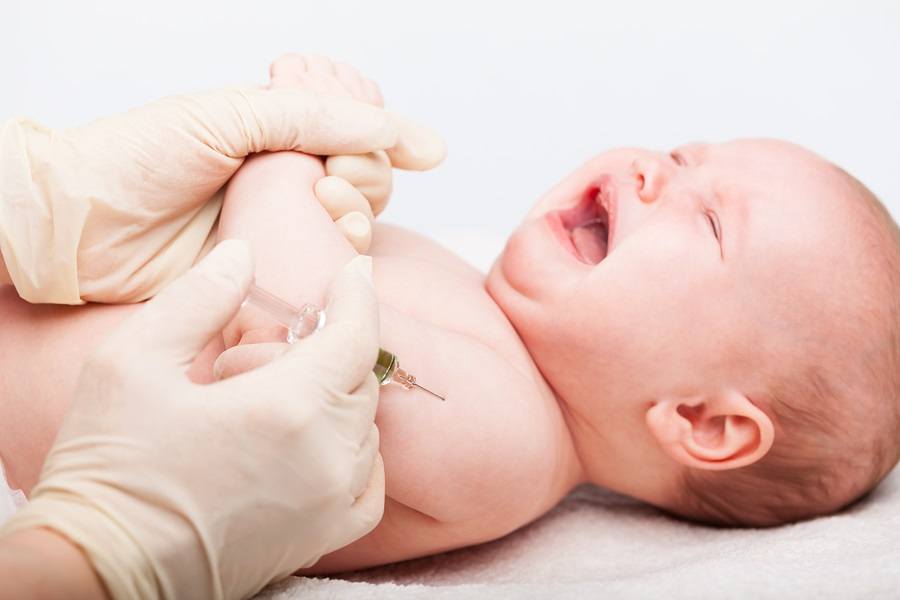
Enduring Mockery, Insults, and Death Threats, Pediatric NICU Nurse Comes Forward to Talk About Her Own Child’s Vaccine Injuries
It’s been too long I have stayed silent. It’s time to share my story. To speak the truth in love. My adult career as a Registered Nurse has been centered on the NICU, PICU, & Pediatric populations. Growing up I had all my vaccines. Fast forward to nursing school. I remember watching a short video, the CDC schedule & the importance of our patients receiving the full schedule. The denial of the autism link - and that’s it. We did not study ingredients, we were not told about vaccine injury/death, or how to report to VAERS when an injury occurred. Upon graduating and securing my first job in the NICU, I thought nothing of the injections I pushed into the thighs of my screaming newborn patients, nor of the “poor feeding,” lethargy, high-pitched screams, & breathing abnormalities that would sometimes follow. This was all normalized as common for the short period after vaccines. I questioned nothing as my own belly grew and grew with my own first child. I was a nurse after all, and this was science. How different things would be had I stood firm and learned back then what I know now. The toxic load of aluminum, formaldehyde, human and animal tissues, etc and etc, were too much for my son’s neurological system and detoxification system. He lost his words & eye contact altogether, started flapping, spinning, walking on his toes, horrible GI symptoms, food limiting, had no desire for social interaction, etc. He was diagnosed with severe autism. And our world was turned upside down. Us pro-vaxxers & Ex-vaxxers are NOT enemies! We all want the same thing - safety for our babies. Do you know why we are suddenly, and in such great numbers, stepping out of hiding, bearing the mockery, insults, wishes of death on us and our children?? It’s because we can’t afford to remain silent any longer, there is TOO much at stake for ALL of us!