by Barbara Loe Fisher
National Vaccine Information Center
Following is a public comment made by Barbara Loe Fisher, NVIC Co-founder & President, at the Nov. 13, 2015 meeting of the FDA Vaccines & Related Biological Products Advisory Committee (VRBPAC) on proposed changes to FDA requirements for licensure of vaccines intended for use during pregnancy
Birth defects, chromosomal damage, premature birth, low birth weight, pregnancy complications and sudden infant death syndrome, not infectious diseases, are the leading causes of death for about 23,000 infants dying before their first birthday in the US every year, with half of those deaths occurring on the first day of life. 1 2 Women getting pregnant and delivering babies in America today have more than twice the risk of dying during pregnancy, childbirth or within one year of giving birth than they did three decades ago, with heart failure, high blood pressure and stroke, diabetes, and blood clots being among the leading causes of death. 3 4
In 2006, CDC officials directed doctors to give all pregnant women a flu shot 5 and, in 2011, a Tdap shot during every pregnancy, no matter how little time has elapsed between pregnancies. 6 Prior to FDA licensure, influenza, diphtheria, tetanus and pertussis vaccines were not tested in or proven safe and effective for pregnant women in large clinical trials when given during every pregnancy either singly or simultaneously. 7 8
Categorized by FDA as Pregnancy Category B and C biologicals 9 because it is not known whether the vaccines are genotoxic and can cause fetal harm or can affect maternal fertility and reproduction, administering influenza and Tdap vaccines to pregnant women is an off-label use of these vaccines. 10 11 12 It is a policy that assumes maternal vaccination is necessary, safe and effective without proving it. 13
Tdap vaccine was licensed by FDA as a single dose pertussis booster shot in individuals over 10 or 11 years old, and pertussis containing vaccine injuries and deaths are the most compensated claim in the federal vaccine injury compensation program (VICP) for infants and children, while influenza vaccine-related injuries and deaths are the most compensated claim for adults. 14 And yet, in the absence of credible biological mechanism and epidemiologic evidence pre-licensure proving these vaccines are safe for all pregnant women, their fetuses and newborns, female health care workers are being fired for refusing to be injected with them while they are pregnant. 15
This maternal vaccination policy is a violation of the precautionary principle to “first, do no harm,” 16 and it is of grave concern to women having babies in America, especially pregnant health care workers. When federal vaccine use recommendations are being turned into laws that punish Americans refusing to obey them with denial of a school education, medical care and employment, 17 18 maintaining high FDA vaccine licensing standards is absolutely essential.
There have been no well designed prospective, long term case controlled studies enrolling large groups of American women who get influenza and Tdap vaccines during pregnancy and comparing their health and the viability of their fetuses and newborns to women who do not get vaccinated. There are no published studies to identify unresolved vaccine-induced inflammation in the brains and bodies of the fetus, mother and newborn; or to measure atypical changes in brain and immune function and chromosomal integrity, including evaluating the numbers of de novo mutations present before and occurring after vaccination.
Maternal vaccination policy has preceded vaccine safety science. Now, there are proposals on the table here in this Committee and in the 21st Century Cures Act backed by FDA and industry to lower FDA licensing standards to ensure that vaccine policy can continue to precede vaccine safety science in the future. 19 Considerable discussion today about making a priori assumptions that complications and fetal death following maternal vaccination are only coincidentally and not causally related to vaccination 20 is a big red flag for vaccine consumers in the absence of credible pathological evidence to conclusively determine what is and is not vaccine induced.
The National Vaccine Information Center is opposed to FDA retroactively licensing influenza and Tdap vaccines for use in pregnant women and fast tracking RSV 21 and group strep B 22 vaccines to licensure by using small clinical trials; adaptive trial designs; Baysian methods of data analysis; biomarkers and surrogate endpoint measures rather than actual clinical endpoints; or using clinical experience instead of good bench science and randomized controlled clinical trials with long term follow-up to prove effectiveness and safety.
The fact that vaccine manufacturers, regulators, policymakers and providers are completely shielded from civil liability for vaccine injuries and deaths 23 makes it even more important for FDA standards for proof of vaccine safety and effectiveness to be very high, especially when licensing vaccines targeting pregnant women and their unborn babies.
References
1 Mathews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/InfantDeath Data Set . National Vital Statistics Reports 2015; 64(9).
2 Manning A. U.S. Top of List for First-Day Deaths in Rich Nations: More Babies Die on Their First Day of Life in the United States Than In Any Other Industrialized Country. National Geographic News May 8, 2013.
3 CDC. Pregnancy Mortality Surveillance System. Trends in Pregnancy-Related Deaths . Sept. 16, 2015.
4 Munz M. Why Are So Many US Women Dying During Childbirth? St. Louis Post Dispatch April 7, 2013.
5 Centers for Disease Control (CDC). Prevention and Control of Influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR July 28, 2006; 55(RR10): 1-42.
6 CDC. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) in Pregnant Women and Persons Who Have or Anticipate Having Close Contact with an Infant Aged Under 12 Months – ACIP, 2011. MMWR 2011; 60(41): 1424-1426.
7 Gruber MF. Maternal Immunization: US FDA Regulatory Considerations . Vaccine 2003; 21(24): 3487-3491.
8 Fisher BL, Wrangham TK. Public Comment to National Vaccine Advisory Committee (NVAC) Maternal Immunization Working Group (MIWG) on MIWG Recommendations on Maternal Immunization . Apr. 25, 2014.
9 Drugs.com. FDA Pregnancy Categories. October 2013.
10 NVIC.org . Influenza Vaccine Package Inserts (2015-2016): Important Information from Manufacturers . NVIC 2013.
11 Sanofi Pasteur. Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed) Full Prescribing Information . Use in Specific Populations: 8.1 Pregnancy Category C. Sanofi Pasteur April 2013.
12 Glaxo-Smith Kline. BOOSTRIX (Tetanus Toxoid, Reducated Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed) Full Prescribing Information . Specific Populations: 8.1 Pregnancy Category B.
13 Fisher BL. Vaccination During Pregnancy: Is It Safe? NVIC Newsletter Nov. 9, 2013.
14 Health Resources Services Administration (HRSA). Vaccine Injury Compensation Program Data & Statistics.
15 CBS News. Pregnant nurse fired for refusing flu shot. Dec. 28, 2013.
16 Science & Environmental Health Network (SEHN). The Wingspread Statement on the Precautionary Principle. SEHN January 1998.
17 Fisher BL.Women, Vaccines & Bodily Integrity. NVIC Newsletter Jan. 24, 2013.
18 Richardson D. The Fallout from California SB277 – What Happens Next? NVIC Newsletter Aug. 5, 2015.
19 Fisher BL. Here Comes the 21st Century Cures Act: Say Goodbye to Vaccine Safety Science . NVIC Newsletter July 21, 2015.
20 FDA. Nov. 13, 2015 VRBPAC Meeting Agenda and FDA Briefing Document: Clinical Development and Requirements for Licensure of Vaccines Intended for Use During Pregnancy to Prevent Disease in the Infant .
21 Fierce Vaccines. Novavax (Nvax) Receives FDA Fast-Track Designation for RSV F Vaccine Candidate . Dec. 4, 2014.
22 Edwards MS, Gonik B. Preventing the broad spectrum of perinatal morbidity and mortality through group B streptococcal vaccination . Vaccine 2013; 31 (Supple 4).
23 Fisher BL. Vaccine Injury Compensation: Government’s Broken Social Contract with Parents. NVIC Newsletter Nov. 2, 2015
Read the full article on National Vaccine Information Center.
Medical Doctors Opposed to Forced Vaccinations – Should Their Views be Silenced?
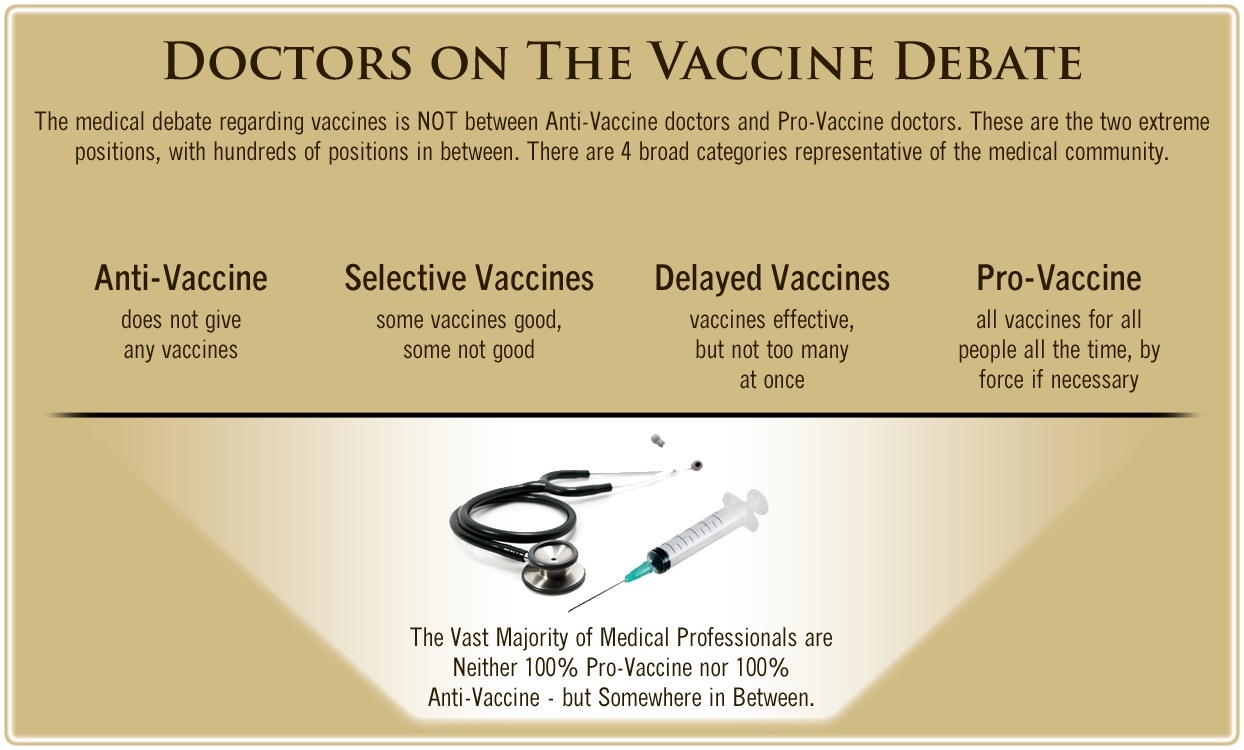
One of the biggest myths being propagated in the compliant mainstream media today is that doctors are either pro-vaccine or anti-vaccine, and that the anti-vaccine doctors are all “quacks.”
However, nothing could be further from the truth in the vaccine debate. Doctors are not unified at all on their positions regarding “the science” of vaccines, nor are they unified in the position of removing informed consent to a medical procedure like vaccines.
The two most extreme positions are those doctors who are 100% against vaccines and do not administer them at all, and those doctors that believe that ALL vaccines are safe and effective for ALL people, ALL the time, by force if necessary.
Very few doctors fall into either of these two extremist positions, and yet it is the extreme pro-vaccine position that is presented by the U.S. Government and mainstream media as being the dominant position of the medical field.
In between these two extreme views, however, is where the vast majority of doctors practicing today would probably categorize their position. Many doctors who consider themselves “pro-vaccine,” for example, do not believe that every single vaccine is appropriate for every single individual.
Many doctors recommend a “delayed” vaccine schedule for some patients, and not always the recommended one-size-fits-all CDC childhood schedule. Other doctors choose to recommend vaccines based on the actual science and merit of each vaccine, recommending some, while determining that others are not worth the risk for children, such as the suspect seasonal flu shot.
These doctors who do not hold extreme positions would be opposed to government-mandated vaccinations and the removal of all parental exemptions.
In this article, I am going to summarize the many doctors today who do not take the most extremist pro-vaccine position, which is probably not held by very many doctors at all, in spite of what the pharmaceutical industry, the federal government, and the mainstream media would like the public to believe.








Leave a Reply