
Project “Bioshield” allows the U.S. Government to stockpile billions of dollars worth of experimental vaccines that have never been approved by the FDA to be used on American Citizens when the government declares a state of emergency.
Brian Shilhavy
Health Impact News Editor
The U.S. Government has guaranteed a thriving U.S. vaccine market for pharmaceutical companies by purchasing billions of dollars worth of vaccines every year with U.S. taxpayer dollars.
As we have previously reported, the CDC (U.S. Government Centers for Disease Control and Prevention) is the largest purchaser of vaccines in the U.S., spending over $4 billion annually to purchase vaccines that are approved by the FDA. Since the CDC is the government agency responsible for overseeing vaccine safety, this creates a huge conflict of interest.
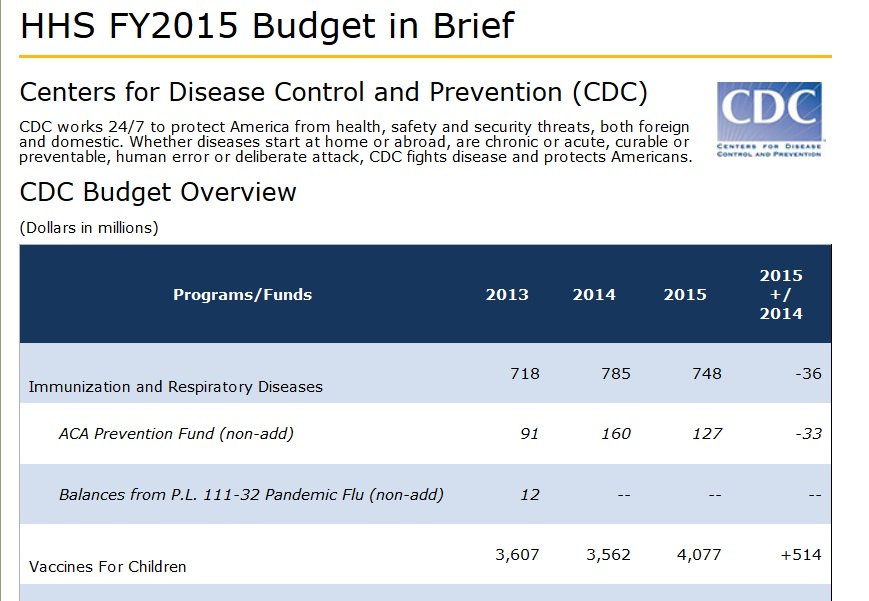
The CDC currently spends over $4 billion purchasing vaccines from drug makers, and wants to increase that amount by over a half billion for 2015. Source.
The U.S. Government guarantees a market for vaccines produced by pharmaceutical companies by legally protecting them from lawsuits in civil court. If anyone is injured or killed by a vaccine, one has to sue the U.S. Government, not the vaccine manufacturers, for compensation. (To learn more about the National Vaccine Injury Compensation Program see: GAO Report on Vaccine Court Reveals Vaccine Injured Victims Not Being Helped.)
Homeland Security Stockpiling Experimental Vaccines
However, this is another U.S. Government agency purchasing billions of dollars’ worth of vaccines. The Department of Homeland Security, created shortly after the terrorist attacks of September 2001, has been authorized by Congress to purchase and stockpile billions of dollars of experimental vaccines that are not approved by the FDA, to use on American citizens during a time of a “national emergency” as defined by the U.S. Government.
The Project Bioshield Act was enacted by Congress in 2004, and signed into law by President George W. Bush. It appropriated $5.6 billion to the Department of Homeland Security “for the purchase of next generation countermeasures against anthrax and smallpox as well as other CBRN agents.”
The last published Project Bioshield Annual Report was for 2013, and is found here. A Congressional report was prepared in June of 2014, and is found here.
Guaranteed Billion Dollar Experimental Vaccine Market by U.S. Government
The Congressional Report prepared by the Congressional Research Service reveals some very troubling facts about Project Bioshield that the general public may not be aware of in regards to taxpayer funded vaccine development and purchases, and the use of such experimental vaccines.
First of all, it guarantees a market for experimental drugs and vaccines where one does not currently exist:
Following the terrorist attacks of 2001, both the Administration and Congress determined that the federal government needed new medical countermeasures (such as diagnostic tests, drugs, vaccines, and other treatments) to respond to an attack using chemical, biological, radiological, or nuclear (CBRN) agents. Representatives of the pharmaceutical industry attributed the paucity of CBRN agent countermeasures to the lack of a significant commercial market.
They argued that, because these diseases and conditions occur infrequently, the private sector perceived little economic incentive to invest the hundreds of millions of dollars required to bring a new treatment to market. In 2004, Congress passed the Project BioShield Act (P.L. 108-276) to encourage the development of CBRN medical countermeasures. The 108th Congress also appropriated $5.6 billion to acquire countermeasures through Project BioShield for FY2004 to FY2013.
Using the authority of the Department of Homeland Security, the U.S. Government can now create a lucrative vaccine market for unapproved experimental vaccines using taxpayer funds, with no conditions of market demand, no competition to keep prices lower, no safety requirements, and no legal risks for faulty products. They instantaneously created another multi-billion dollar vaccine market to supplement the already existing billion dollar vaccine market for approved vaccines:
Project BioShield contracts can have multiple benefits to potential CBRN medical countermeasure developers. The contracts can reduce market risk by defining the minimum economic value of a product. They can also mitigate development risk and provide some revenue during product development through milestone-based payments.
A key factor for companies deciding whether to develop a new product is its potential economic value. The U.S. government may be the most economically significant customer for new CBRN countermeasures. The federal government maintains the Strategic National Stockpile (SNS), a supply of pharmaceuticals, vaccines, medical supplies, and medical equipment to respond to terrorist attacks and other emergencies. Thus, one difficulty facing potential CBRN developers is knowing whether the federal government would buy their product for the SNS and, if so, at what price.
Under Project BioShield, HHS may purchase unapproved and unlicensed countermeasures.
(Source: The Project BioShield Act: Issues for the 113th Congress)
When Can Unapproved Vaccines and Drugs be Used on American Citizens?
One might think that the only time an unapproved vaccine or drug could be authorized for use would be during a time of national crisis, such as a biological terrorist attack. The fact is, however, that the government has broad powers to use unapproved vaccines and drugs whenever they want to, and have already done so.
In the Project Bioshield Annual report for 2013, the “Emergency Use Authorization” is defined:
Section 564 of the FD&C Act (21 U.S.C. 360bbb-3), as enacted under the PBS Act and amended under PAHPRA, enables the Commissioner of FDA to issue an EUA to authorize the use of an unapproved medical product, or to authorize an unapproved use of an approved medical product, when the HHS Secretary determines that an EUA is justified based on one of four declarations or determinations by the Secretaries of Homeland Security, Defense, or HHS.
Since 2004, FDA has exercised its EUA authority numerous times to facilitate preparedness for and response to emergencies.
(Emphasis added.)
Experimental Anthrax Vaccine Used on U.S. Military in 2005
According to the Project Bioshield report, the first time the FDA used their authority to administer an unapproved vaccine on American citizens was during 2005, when it forced U.S. military personnel to receive an experimental anthrax vaccine. The FDA apparently had to use their authority under Project Bioshield, because there was a civil lawsuit at the time challenging the Department of Defense’s authority to mandate unapproved vaccines to military personnel:
FDA’s first use of its EUA authority was in 2005, when the Agency issued an EUA to enable the use of Anthrax Vaccine Adsorbed (AVA) in military personnel deemed by DoD to be at heightened risk of exposure due to a potential attack with Bacillus anthracis, the causative agent of anthrax.
FDA issued this EUA to enable the continued use of AVA under the DoD Anthrax Vaccine Immunization Program (AVIP) while a challenge to FDA’s ruling that AVA is safe, effective, and not misbranded for the prevention of inhalational anthrax was addressed as part of a civil suit over the legality of mandatory vaccinations under the AVIP. This EUA is no longer in effect.
While this particular “Emergency Use Authorization” order is no longer in effect, others regarding stockpiling of experimental and unapproved anthrax medications are still in effect:
FDA has also issued two EUAs to facilitate pre-event planning, stockpiling, and, if necessary, rapid response efforts in support of preparedness for an anthrax attack. Both EUAs remain in effect and authorize certain unapproved uses of the antimicrobial drug doxycycline for post-exposure prophylaxis of inhalational anthrax in the event of a public health emergency involving Bacillus anthracis.
The first of these EUAs, issued in 2011, is focused on facilitating stakeholder mass dispensing activities by authorizing the use of all approved oral formulations of doxycycline products (where not contraindicated) for the post-exposure prophylaxis of inhalational anthrax. The EUA authorizes certain aspects of emergency stockpiling, distribution, dispensing, and use of oral formulations of doxycycline products that otherwise might violate provisions of the FD&C Act.
For example, in the event of an anthrax emergency, this EUA allows public health authorities to mass dispense doxycycline at points of dispensing with emergency use information (e.g., streamlined fact sheets for recipients about how to use the product) that is not part of the product’s approved labeling, without individual prescriptions, and by responders and volunteers who are not licensed health care professionals. (Source.)
(Emphasis added. Might these “responders” who are “not licensed health care professionals” be military and law enforcement officers administering these unapproved drugs and vaccines by force?)
Flu Drugs that were Unapproved and Authorized by The Project Bioshield Act
While potential acts of bio-terrorism are used as a rationale to authorize the use of unapproved vaccines and drugs on American citizens, the 2013 Bioshield Annual Report states that the authority was used during the 2009 flu season:
FDA used its EUA authority extensively in 2009 – 2010 to facilitate the nation’s response to the 2009 H1N1 influenza pandemic including issuing three EUAs to enable the use of antiviral medications for the treatment and/or prophylaxis of influenza; one EUA to enable the use of certain N95 respirators to help reduce wearer exposure to pathologic biologic airborne particulates; and 18 EUAs to enable the use of in vitro diagnostic tests for the diagnosis of 2009 H1N1 influenza virus infection.
Is the 2015 Measles Hype a Precursor to Expand Vaccine Market Via Government Mandated Vaccines?
As we have shown, the U.S. Government has guaranteed pharmaceutical companies a market for both their approved and unapproved experimental vaccines. It is a market worth billions of dollars, guaranteed by the U.S. Government, who purchases most of these vaccines with U.S. taxpayer funds. The pharmaceutical companies bear no risk whatsoever, as they cannot be sued in civil court for faulty products that cause injury and death.
How could there be a better market with such guaranteed results? What could possibly stand in the way of such a powerful market?
Vaccine objectors.
The current U.S. population is the most heavily vaccinated population in the world, but apparently the pharmaceutical lobbyists and the U.S. Government are not satisfied. All across the United States right now, at both the local and federal level, politicians are proposing new legislation to remove vaccine exemptions and pursue mandatory vaccines.
And this is largely based on the fact that just over 100 people have been infected with the measles virus this year, with no deaths. If one were to create a table of the top diseases and illnesses infecting Americans in 2015, would measles even be in the top 100?
It would certainly appear that there is something else going on besides a genuine concern about measles infections.
As the current vaccine debate is being carried out all across the U.S., the current City Council dispute happening in Spokane Washington is probably representative of what is happening all across the country, where politicians with little or no medical or legal background are attempting to change public policy on health laws by mandating vaccines or squelching free speech by vaccine objectors.
In Spokane, they currently have a Health Board member who was “editor-in-chief and contributor for the Star Wars fan magazine Star Wars Insider as well as the Star Wars Kids magazine,” and a first term City Council president who previously worked in “customer service and management with the Oakland Raiders and TicketsWest,” trying to set public policy on vaccines and silence a fellow Council member on the Board of Health who is “a disabled American veteran, who served as a criminal investigator and contracting officer in the Army” by removing him from the Board of Health. (Story here.)
Wake up America! If you don’t want politicians taking away your health freedoms and your ability to refuse medical treatments and drugs, including experimental ones not even approved by the FDA, it is time to make your voice heard, whether you are pro-vaccine, anti-vaccine, or somewhere in between.
As for the current political dispute about funding the Department of Homeland Security, tell your Congressional representative and Senators to defund or repeal the Project Bioshield Act as a starter.
Medical Tyranny is alive and well in the United States, and the only hope we have to reverse this trend is an informed and educated public that resists.
Visit our Resources Page where you can learn about existing laws regarding vaccines, and sign up for updates in your state regarding efforts attempting to remove your rights to informed consent.

Free Shipping Available! Order here.




8 Comments