by David Michael
Journal of Natural Food and Health
Drugs called Tamiflu are chemicals (ethyl (3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)-cyclohex-1-ene-1-carboxylate) that are said to prevent flu or treat the flu. This CDC-approved and recommended-for-all chemotherapy has been shown to cause more vomiting and diarrhea. Roche, the maker, has refused to release the data from studies on the effectiveness of Tamiflu to independent researchers for over three years.
Chemotherapy? Yes, according to the medical definition at dictionary.com (and others), chemotherapy is the treatment of disease by means of chemicals that have a specific toxic effect upon the disease-producing microorganisms or that selectively destroy cancerous tissue. So technically this is what Tamiflu therapy is.
Prevention? If you were given a $120 dollar bottle of mints and told it would prevent the flu– it would seem to work 90% of the time. This is because, on average, you already have a 90% chance of not getting the flu, give or take. If you still get the flu you are told you were infected before starting the therapy but the mints have made your flu milder than it would have been. You may be told a few people will still get the flu during or after eating the mints because mints are not 100% effective, but if you don’t take it you have no protection at all.
Treatment? Let’s say you have the flu already for a couple days . CDC says to hurry in as soon as flu-like symptoms appear. The doctor gives you the mints and tells you will eventually feel better and the flu will go away. Sure enough you recover. You will start to feel better the next day or two–as would with folks who just stayed home, rested and drank plenty of water.
But Tamiflu are not mints, they are a drug– and all drugs have side effects. However it does appear you will be better off taking mints rather than Tamiflu.
What are the Benefits of Tamiflu?
The Tami in Tamiflu is of Japanese origin, and the meaning of Tami is “let people see benefit”. So, let’s look at the benefits of ethyl (3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)-cyclohex-1-ene-1-carboxylate.
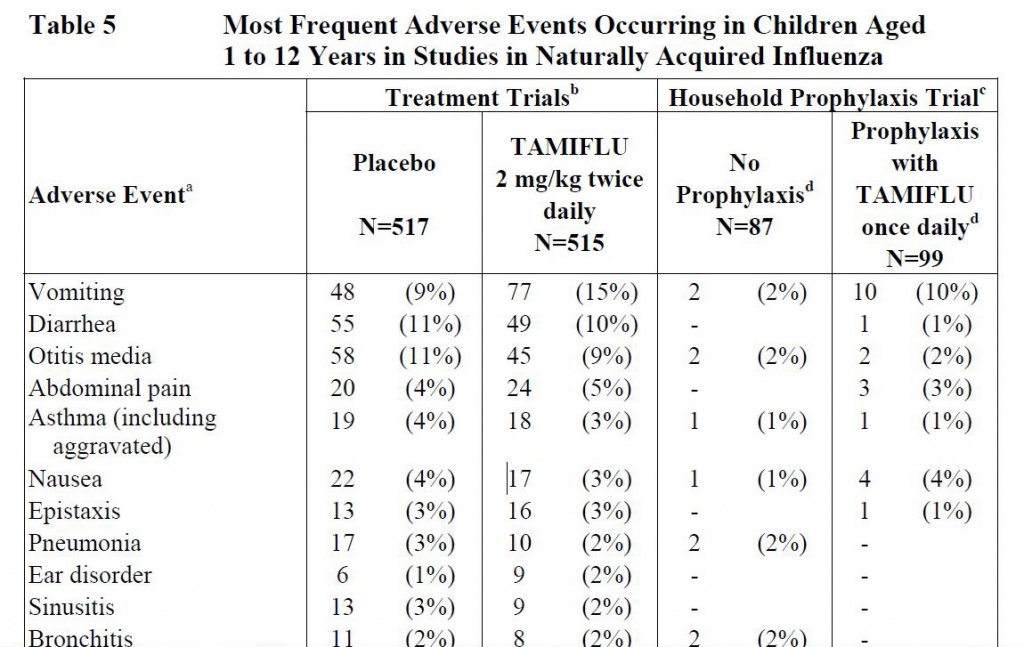
The extract of the Roche report below shows 1 to 12 year olds being treated with Tamiflu for flu have about the same symptoms as those taking a placebo (sugar pill)– except 70% more kids were vomiting after Tamiflu than taking nothing (an increase from 9% to 15% is a 70% increase–not 6%) of the kids. Five times more children taking Tamiflu for flu prevention had vomiting and four times more kids had nausea than those who took nothing. In other words, Tamiflu makes kids sicker.
Even so, CDC recommends that infants as young as two weeks receive Tamiflu and they are clear that Tamiflu is not a substitute for flu vaccines and that flu vaccinations should be taken also.
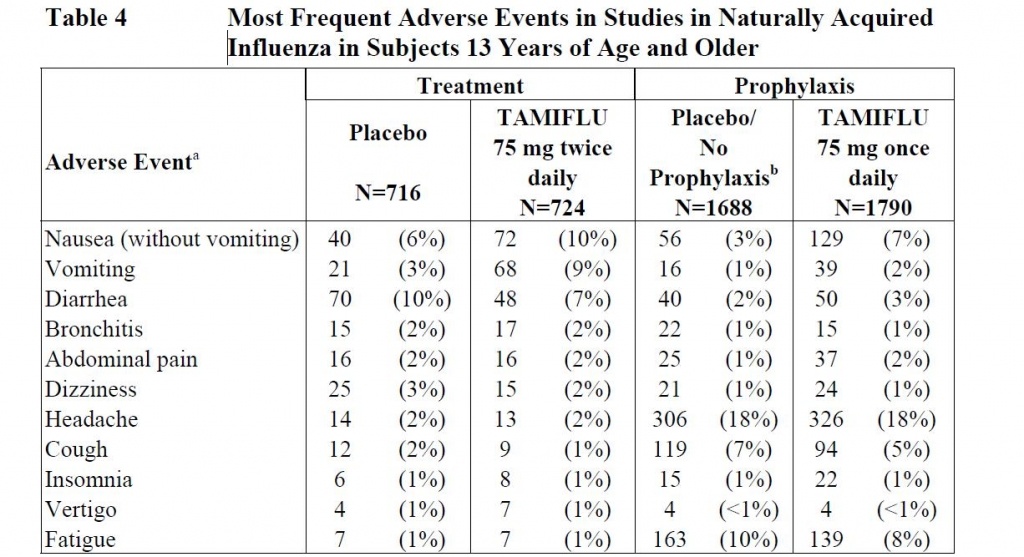
What about the adverse reactions for teenagers and adults? According to their own clinical trial, the group being treated for flu has nearly 2x the nausea, 3x the vomiting and about the same other flu symptoms as those taking nothing (except for a little less diarrhea). Those taking Tamiflu for 5 weeks as recommended for flu prevention had 2x more had nausea, 2x more had vomiting, 2x more had stomach pains and 50% more had diarrhea than those taking nothing. In other words, Tamiflu made them sicker.
The Roche report states no studies have been conducted on the safety of Tamiflu for pregnant women or fetuses and is not to be taken (unless the doctor decides the benefits outweigh the risks). Yet CDC says that pregnant women should rush out and take it at the first signs of flu.
CDC explains that onset of mental conditions, brain swelling and death may occur after taking Tamiflu but assures us that the cause has not been established. We are further assured that most of these cases have only been seen in Japan.
How effective is Tamiflu in preventing or treating the flu? There are no statements or data given concerning its effectiveness on the Roche report. A search of the CDC website did not show anything regarding how well it works. According to this report on a British Medical Journal article, Roche has refused to hand over clinical trial data on the effectivess of Tamiflu. Quote,
In 2009, the BMJ and researchers at the Nordic Cochrane Centre asked Roche to make all its Tamiflu data available. At the time, Cochrane Centre scientists were commissioned by Britain to evaluate flu drugs. They found no proof that Tamiflu reduced the number of complications in people with influenza. As of January 2013 the data has not been released to them.
Roche is also being investigated by the European Medicines Agency for not properly reporting side effects, including possible deaths, for 19 drugs including Tamiflu that were used in about 80,000 patients in the U.S.
The two brief videos (one is a BBC headline news broadcast from 2009 when this deception was uncovered and the other is a doctor and nurse produced last month).
Read the full article here: http://journal.livingfood.us/2013/02/02/the-tamiflu-deception/
Saying NO To Vaccines
By Dr. Sherri Tenpenny
You have legal options!