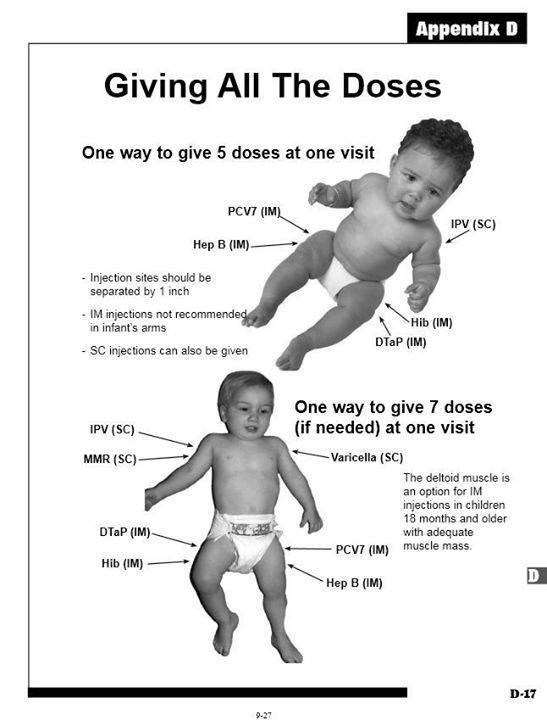
Page from a pediatrician manual showing how to administer multiple vaccines at one doctor’s office visit.
Comments by Brian Shilhavy
Editor, Health Impact News
One of the great tragedies of modern times is the near total censorship in the mainstream media of any information that casts a negative light on vaccines. As we have reported numerous times here at Health Impact News, the “science” related to vaccines is far from “settled,” and medical doctors are not unified in their opinions regarding vaccines.
From the two extremist positions, ranging from doctors who do not believe in administering any vaccines at all, to those doctors who believe that all vaccines are safe and should be administered to everyone, by force if necessary, there are numerous doctors who fall in between these two extreme positions. Some physicians believe in a delayed vaccine schedule, some believe in single doses and not multiple doses, and others accept certain vaccines while rejecting others, evaluating each vaccine on its own merit and appropriateness for their patients. (For more on this topic, see: Medical Doctors Opposed to Forced Vaccinations – Should Their Views be Silenced?)
Yet, it is only the extremist position that all vaccines are safe and appropriate for everyone, by force if necessary, that is presented by mainstream media and the U.S. Government. The multi-billion dollar pharmaceutical vaccine business obviously exerts tremendous influence on the media and government policy.
Neil Miller has published an excellent study in Journal of American Physicians and Surgeons on the effects of administering multiple vaccines to a child at one doctor’s office visit. The CDC themselves admit that no safety studies have been conducted on multiple dose vaccines.
Combining Childhood Vaccines at One Visit Is Not Safe
by Neil Z. Miller
Journal of American Physicians and Surgeons, Volume 21, Number 2, Summer 2016
ABSTRACT
Although health authorities including the Centers for Disease Control and Prevention (CDC) claim that childhood vaccines are safe and recommend combining multiple vaccines during one visit, a review of data from the Vaccine Adverse Event Reporting System (VAERS) shows a dose-dependent association between the number of vaccines administered simultaneously and the likelihood of hospitalization or death for an adverse reaction. Additionally, younger age at the time of the adverse reaction is associated with a higher risk of hospitalization or death.
Background
In the 1980s vaccine manufacturers were frequently sued by the parents of children who were permanently disabled or died following vaccination. After paying out millions of dollars in these lawsuits, vaccine manufacturers were prepared to stop producing vaccines unless the federal government provided them with immunity from jury verdicts.
In response to pharmaceutical manufacturers’ threat to close their own vaccine factories, in 1986 Congress passed the National Childhood Vaccine Injury Act (NCVIA), protecting vaccine manufacturers from most financial liability associated with their products. Under NCVIA, the National Vaccine Injury Compensation Program (VICP) was created to provide cost-effective arbitration for vaccine injury claims. Vaccine manufacturers can no longer be sued in a state or federal court for damages arising from a vaccine-related injury or death unless a petition for compensation under the new program is filed and denied.
Compensation under the program is paid for by a 75-cent excise tax on every vaccine purchased. (MMR contains three vaccines, so the tax is $2.25.) The money goes into a Trust Fund managed by the U.S. Department of the Treasury. As of Mar 1, 2016, more than $3.2 billion had already been paid out, most of it to compensate parents whose children were severely disabled or died after receiving vaccines. [1] Today, vaccine manufacturers not only make millions of dollars annually from their lucrative business, but they have been disincentivized from producing safer vaccines, since they are shielded from liability when their mandatory products harm consumers.
Vaccine Adverse Event Reporting System (VAERS)
The new federal law also required medical workers to report suspected vaccine reactions to a centralized reporting system. As a result, the Vaccine Adverse Event Reporting System (VAERS), jointly operated by CDC and the U.S. Food and Drug Administration (FDA), was established in 1990. VAERS is a national vaccine safety surveillance program that collects information about possible adverse reactions to vaccines. This large database is accessible to the general public, including independent researchers who may use it to look for patterns in the data that might indicate vaccine safety concerns or problems. [2]
VAERS is a passive surveillance system, which means that reports about adverse events are not automatically collected. VAERS relies on doctors and nurses to voluntarily submit reports, although vaccine recipients and parents may also file reports. Vaccine manufacturers are required to report all adverse events of which they become aware. Since 1990, the VAERS database has received more than 500,000 reports of suspected adverse reactions to vaccines. Although this represents a large number of people who may have been hurt by vaccines, under-reporting is a known limitation of passive surveillance systems. This means that VAERS only captures a small fraction of actual adverse events. In fact, shortly after VAERS was established, a large vaccine manufacturer, Connaught Laboratories, estimated “about a 50- fold under-reporting of adverse events in the passive reporting system.” [3] Perhaps 98% of all adverse reactions to vaccines are not included in the VAERS database, and up to 25 million U.S. citizens could have been adversely affected by vaccines in the past 25 years. This well-known disadvantage of a passive reporting system, as opposed to an active surveillance system in which medical workers are trained to systematically collect all cases of suspected adverse vaccine reactions, is rarely acknowledged by health authorities when vaccine safety is discussed.
Although VAERS collects information about adverse events that occur after vaccines are administered, it should be noted that a report is not a confirmation that a vaccine caused the event. Health authorities like to emphasize this point whenever VAERS data are used in a study with findings that are critical of vaccines. The implication is that studies using VAERS are unreliable and should be disregarded. However, CDC considers VAERS an important vaccine safety assessment tool and regularly conducts its own studies using VAERS data, often to justify maintaining national vaccination campaigns.
CDC Studies Utilizing VAERS
In May 2015, the CDC published a study in Clinical Infectious Diseases that analyzed the VAERS database for reports of serious adverse events after MMR vaccination in adults. CDC researchers found that the vaccine was often administered to pregnant women, a group in whom the vaccine is contraindicated, “suggesting the need for continued provider education on vaccine recommendations and screening.” Although 5% of reports were serious, including several deaths, CDC researchers concluded that “in our review of VAERS data, we did not detect any new or unexpected safety concerns for MMR vaccination in adults.” [4]
In November 2014, CDC published a study in the journal Vaccine that analyzed VAERS reports associated with the live attenuated influenza vaccine (LAIV3). Although 8.9% of reports were classified as serious (e.g., cardiovascular events, neurological debilities, and fatalities) CDC researchers concluded that “review of VAERS reports are reassuring, the only unexpected safety concern for LAIV3 identified was a higher than expected number of Guillain-Barré syndrome reports in the Department of Defense population, which is being investigated [sic].” [5]
In June 2013, the CDC published a study in the journal Pediatrics that analyzed the VAERS database to assess intussusception events in recipients of two rotavirus vaccines, RotaTeq and Rotarix. (Intussusception is a serious intestinal condition that may require emergency surgery and can be fatal.) Although there were hundreds of confirmed intussusception events after vaccination, and a statistically significant clustering of intussusception events 3 to 6 days after the first dose of RotaTeq vaccination, CDC researchers concluded that an increased risk of intussusception “is outweighed by the benefits of rotavirus vaccination.” [6]
These studies and others confirm that CDC considers VAERS an important post-marketing vaccine safety surveillance tool. Therefore, nobody should be swayed into believing the VAERS database does not contain immensely valuable raw data to be used by independent researchers conducting studies that evaluate the safety of U.S. mandated vaccines. For example, Mark Geier, M.D., Ph.D., independent researcher and former professional staff member at the National Institutes of Health (NIH), published several studies utilizing the VAERS database showing that vaccines containing thimerosal (mercury) significantly increase the odds of developing neurological disorders, including autism. [7-9] Independent researchers Lai and Yew utilized the VAERS database and discovered that patients who received a Herpes zoster (shingles) vaccine were more than twice as likely to subsequently develop arthritis or alopecia compared to a non- vaccinated control group. [10] Other independent researchers have used VAERS to document numerous vaccine safety concerns; some of their peer-reviewed papers are summarized in Miller’s Review of Critical Vaccine Studies. [11]
The Safety of Simultaneous Vaccines
Although CDC recommends polio, hepatitis B, diphtheria, tetanus, pertussis, rotavirus, Haemophilus influenzae type B, and pneumococcal vaccines for two-, four-, and six-month-old infants, this combination of eight vaccines administered during a single physician visit was never tested for safety in clinical trials. This is at odds with a CDC report that found that mixed exposures to chemical substances and other stress factors, including prescribed pharmaceuticals, may produce “increased or unexpected deleterious health effects.” This CDC report also noted that “exposures to mixed stressors can produce health consequences that are additive, synergistic, antagonistic, or can potentiate the response expected from individual component exposures.” [12] Thus, CDC is well aware that mixing several pharmaceutical products increases the likelihood of synergistic toxicity and unexpected adverse reactions. Nonetheless, CDC urges infants to receive multiple vaccines concurrently without scientific evidence to confirm the safety of this practice. Administering six, seven, or eight vaccine doses to an infant during a single physician visit is certainly more convenient for parents, as opposed to making additional trips to the doctor’s office, and increases the likelihood that the infant will receive all the vaccines, but vaccine safety must remain the highest priority.
In 2002, the journal Pediatrics published a paper by Dr. Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, in which he claimed that based upon certain immunological and mathematical assumptions, “each infant would have the theoretical capacity to respond to about 10,000 vaccines at any one time.” [13] Ten years later, in 2012, G.S. Goldman and I conducted a study that examined this astonishing claim. [14]
We started by downloading the complete VAERS database from 1990 through 2010. There were more than 325,000 VAERS
reports. We then eliminated all case reports that were not associated with infants (babies aged up to one year). This left us with 38,801 VAERS reports in which infants had adverse events after receiving one or more vaccine doses.
Next, we determined how many vaccine doses each infant received prior to the adverse event. (A computer program was written to make these calculations.) For example, if an infant received a hepatitis B vaccine and a rotavirus vaccine prior to the adverse event, it was recorded as two vaccine doses. DTaP is administered with one injection but contains three separate vaccine doses, for diphtheria, tetanus, and acellular pertussis. Thus, if an infant received a polio vaccine, a pneumococcal vaccine, and DTaP prior to the adverse event, it was recorded as five vaccine doses. Some babies received six, seven, or eight doses prior to an adverse event. This was not unusual because of the CDC recommendations noted above, plus its recommendation for two doses of an influenza vaccine during infancy.
Finally, we isolated the “serious” adverse events— hospitalizations and death—from non-serious events, such as fever and local reactions. About 13% of all adverse events reported to VAERS are classified as serious, involving life- threatening conditions, hospitalization, permanent disability, or death. We sought to determine whether there were any trends or patterns associated with the number of vaccine doses an infant received and the likelihood that the adverse event reported to VAERS would require hospitalization or result in death.
Vaccine Doses and Hospitalizations
Of the 38,801 VAERS reports that we analyzed, 969 infants received two vaccine doses prior to the adverse event and 107 of those infants were hospitalized: a hospitalization rate of 11%. Of 1,959 infants who received three vaccine doses prior to the adverse event, 243 of them required hospitalization: 12.4%. For four doses, 561 of 3,909 infants were hospitalized: 14.4%. Notice the emerging pattern: Infants who had an adverse event reported to VAERS were more likely to require hospitalization when they received three vaccine doses instead of two, or four vaccine doses instead of three.
The pattern continues: Of 10,114 infants who received five vaccine doses prior to the adverse event, 1,463 of them required hospitalization: 14.5%. For six doses, 1,365 of 8,454 infants were hospitalized: 16.1%. For seven doses, 1,051 of 5,489 infants were hospitalized: 19.1%. And for eight doses, 661 of 2,817 infants were hospitalized: 23.5%. The hospitalization rate increased linearly from 11.0% for two doses to 23.5% for eight doses. Linear regression analysis of hospitalization rates as a function of the number of reported vaccine doses yielded a linear relationship, with an R2 of 0.91.
Note: The hospitalization rate of infants who received just one vaccine dose was disproportionately high (16.3%) due to the hepatitis B vaccine administered at birth. As such, the hospitalization rate corresponding to one dose is an outlier and was excluded from the linear regression analysis.
Vaccine Doses and Mortality
Our study also calculated the case fatality ratio (mortality rate) among vaccinated infants, stratified by the number of vaccine doses they received. Of the 38,801 VAERS reports that we analyzed, 11,927 infants received one, two, three, or four vaccine doses prior to having an adverse event, and 423 of those infants died: a mortality rate of 3.6%. The remaining 26,874 infants received five, six, seven, or eight vaccine doses prior to the adverse event and 1,458 of them died: 5.4%. The mortality rate for infants who received five to eight vaccine doses (5.4%) is significantly higher than the mortality rate for infants who received one to four vaccine doses (3.6%), with a rate ratio (RR) of 1.5 (95% CI, 1.4-1.7). Of infants reported to VAERS, those who had received more vaccines had a statistically significant 50% higher mortality rate compared with those who had received fewer.
The Age Effect on Hospitalizations and Death
Our study also analyzed whether the age at which an infant received vaccines had an effect on hospitalizations and death. Of the 38,801 VAERS reports that we analyzed, 765 concerned infants six-weeks-old or younger who received one or more vaccine doses prior to the adverse event, and 154 of those infants were hospitalized: a hospitalization rate of 20.1%. Of 5,572 infants aged six months at vaccination, 858 were hospitalized: 15.4%. Of 801 infants who were nearly a year old when they were vaccinated, 86 were hospitalized: 10.7%. The hospitalization rate decreased linearly from 20.1% for neonates to 10.7% for older infants. Linear regression analysis of hospitalization rates as a function of patient age yielded an R2 of 0.95.
In the 38,801 VAERS reports we analyzed, 26,408 infants were younger than six months. After receiving one or more vaccine doses, 1,623 of those infants died: a mortality rate of 6.1%. The remaining 12,393 infants were between six months and one year of age. After receiving one or more vaccine doses, 258 of them died: 2.1%. The mortality rate for vaccinated infants younger than six months was significantly higher than the mortality rate for vaccinated infants aged between six months and one year, with an RR = 3.0 (95% CI, 2.6-3.4). Infants who had an adverse event reported to VAERS were significantly more likely to be hospitalized or die if they were younger rather than older at the time of vaccination.
Summary of Results and Media Response
Our study showed that infants who receive several vaccines concurrently, as recommended by CDC, are significantly more likely to be hospitalized or die when compared with infants who receive fewer vaccines simultaneously. It also showed that reported adverse effects were more likely to lead to hospitalization or death in younger infants.
These findings are so troubling that we expected major media outlets in America to sound an alarm, calling for an immediate reevaluation of current preventive health care practices. But 4 years after publication of our study, this has not happened. Could it be because, according to Robert Kennedy, Jr., about 70% of advertising revenue on network news comes from drug companies? In fact, the president of a network news division admitted that he would fire a host who brought on a guest that led to loss of a pharmaceutical account. That may be why the mainstream media won’t give equal time to stories about problems with vaccine safety. [15]
Conclusion
The safety of CDC’s childhood vaccination schedule was never affirmed in clinical studies. Vaccines are administered to millions of infants every year, yet health authorities have no scientific data from synergistic toxicity studies on all combinations of vaccines that infants are likely to receive. National vaccination campaigns must be supported by scientific evidence. No child should be subjected to a health policy that is not based on sound scientific principles and, in fact, has been shown to be potentially dangerous.
Undesirable outcomes associated with childhood vaccination can be reduced by requiring national vaccination policies to be supported by scientific evidence, holding vaccine manufacturers accountable when their products harm consumers, and urging major news outlets that rely on pharmaceutical advertising revenue to change their business models so that crucial scientific research, regardless of how controversial it may be, is widely disseminated into the public domain. Meanwhile, the evidence presented in this study shows that multiple vaccines administered during one visit, and vaccinating young infants, significantly increase morbidity and mortality. Parents and physicians should consider health options associated with a lower risk of hospitalization or death.
Neil Z. Miller is a medical research journalist. Contact: neilzmiller@gmail.com. Disclosures: No conflicts of interest were disclosed.
See Also:
Medical Doctors Opposed to Forced Vaccinations – Should Their Views be Silenced?
REFERENCES
1. U.S. Department of Health and Human Services. National Vaccine Injury Compensation Program. Available at: http://www.hrsa.gov/ vaccinecompensation. Accessed Feb 14, 2016.
2. U.S. Department of Health and Human Services. Vaccine Adverse Event Reporting System (VAERS). Available at: https://vaers.hhs.gov. Accessed Feb 14, 2016.
3. Institute of Medicine (U.S.) Vaccine Safety Committee. Appendix B: Strategies for Gathering Information. In: Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. Stratton KR, Howe CJ, Johnston RB Jr., eds. Washington, D.C.: National Academies Press (U.S.); 1994. Available at: http://www.ncbi.nlm.nih.gov/books/NBK236281. Accessed Mar 5, 2016.
4. Sukumaran L, McNeil MM, Moro PL, et al. Adverse events following measles, mumps, and rubella vaccine in adults reported to the Vaccine Adverse Event Reporting System (VAERS), 2003-2013. Clin Infect Dis 2015;60(10):e58-65.
5. Haber P, Moro PL, McNeil MM, et al. Post-licensure surveillance of trivalent live attenuated influenza vaccine in adults, United States, Vaccine Adverse Event Reporting System (VAERS), July 2005-June 2013. Vaccine 2014;32(48):6499-6504.
6. Haber P, Patel M, Pan Y, et al. Intussusception after rotavirus vaccines reported to U.S. VAERS, 2006-2012. Pediatrics 2013;131(6):1042-1049.
7. Geier DA, Hooker BS, Kern JK, et al. A two-phase study evaluating the relationship between thimerosal-containing vaccine administration and the risk for an autism spectrum disorder diagnosis in the United States. Transl Neurodegener 2013;2(1):25.
8. Geier DA, Kern JK, King PG, Sykes LK, Geier MR. The risk of neurodevelopmental disorders following a thimerosal-preserved DTaP formulation in comparison to its thimerosal-reduced formulation in the Vaccine Adverse Event Reporting System (VAERS). J Biochem Pharmacol Res 2014;2(2):64-73.
9. Geier DA, Geier MR. An assessment of the impact of thimerosal on childhood neurodevelopmental disorders. Pediatr Rehabil 2003;6(2):97-102.
10. Lai YC, Yew YW. Severe autoimmune adverse events post Herpes zoster vaccine: a case-control study of adverse events in a national database. J Drugs Dermatol 2015;14(7):681-684.
11. Miller NZ. Miller’s Review of Critical Vaccine Studies: 400 Important Scientific Papers Summarized for Parents and Researchers. Santa Fe, N.M.: New Atlantean Press; 2016.
12. Castranova V, Graham J, Hearl F, et al. Mixed exposures research agenda: a report by the NORA Mixed Exposures Team. Department of Health and Human Services (DHHS), Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH). DHHS (NIOSH) Publication No. 2005-106; December 2004:vi. Available at: http:// www.cdc.gov/niosh/docs/2005-106/pdfs/2005-106.pdf. Accessed Feb 14, 2016.
13. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 2002;109(1):124-129.
14. Goldman GS, Miller NZ. Relative trends in hospitalizations and mortality among infants by the number of vaccine doses and age, based on the Vaccine Adverse Event Reporting System (VAERS), 1990-2010. Hum Exp Toxicol 2012;31(10):1012-1021. Available at: http://het.sagepub.com/ content/31/10/1012.full. Accessed Feb 14, 2016.
15. Jaxen, J. Kennedy drops bombshell: 70% news ad revenue from pharma. Before It’s News, May 22, 2015. Available at: http://beforeitsnews.com/ health/2015/05/kennedy-drops-bombshell-70-news-ad-revenue-from- pharma-2574590.html. Accessed Feb 14, 2016.
Read the full report at jpands.org
Medical Doctors Opposed to Forced Vaccinations – Should Their Views be Silenced?
One of the biggest myths being propagated in the compliant mainstream media today is that doctors are either pro-vaccine or anti-vaccine, and that the anti-vaccine doctors are all “quacks.”
However, nothing could be further from the truth in the vaccine debate. Doctors are not unified at all on their positions regarding “the science” of vaccines, nor are they unified in the position of removing informed consent to a medical procedure like vaccines.
The two most extreme positions are those doctors who are 100% against vaccines and do not administer them at all, and those doctors that believe that ALL vaccines are safe and effective for ALL people, ALL the time, by force if necessary.
Very few doctors fall into either of these two extremist positions, and yet it is the extreme pro-vaccine position that is presented by the U.S. Government and mainstream media as being the dominant position of the medical field.
In between these two extreme views, however, is where the vast majority of doctors practicing today would probably categorize their position. Many doctors who consider themselves “pro-vaccine,” for example, do not believe that every single vaccine is appropriate for every single individual.
Many doctors recommend a “delayed” vaccine schedule for some patients, and not always the recommended one-size-fits-all CDC childhood schedule. Other doctors choose to recommend vaccines based on the actual science and merit of each vaccine, recommending some, while determining that others are not worth the risk for children, such as the suspect seasonal flu shot.
These doctors who do not hold extreme positions would be opposed to government-mandated vaccinations and the removal of all parental exemptions.
In this article, I am going to summarize the many doctors today who do not take the most extremist pro-vaccine position, which is probably not held by very many doctors at all, in spite of what the pharmaceutical industry, the federal government, and the mainstream media would like the public to believe.





One Comment