Can Measles Vaccine Cause Injury & Death?
by the National Vaccine Information Center
The Centers for Disease Control (CDC) report minor side effects from the MMR-V and MMR vaccines to include low-grade fever, injection site redness or rash, pain at the injection site, and facial swelling. Moderate side effects include a full body rash, temporary low platelet count, temporary stiffness and pain the joints and seizures, and seizures. [1] [2]
MMR-V, however, has been noted to have a higher risk of seizures than separate administrations of MMR and varicella vaccines, especially when given as the first dose of the series.[3]
Rare serious side effects of both MMR-V and MMR include brain damage, coma, chronic seizure disorder, lowered level of consciousness and loss of hearing.[4] [5]
Serious complications reported by Merck in the ProQuad (MMR-V) product insert during vaccine post-marketing surveillance include[6]:
- measles;
- atypical measles;
- vaccine strain varicella;
- varicella-like rash;
- herpes zoster;
- herpes simplex;
- pneumonia and respiratory infection;
- pneumonitis;
- bronchitis;
- epididymitis;
- cellulitis;
- skin infection;
- subacute sclerosing panencephalitis;
- aseptic meningitis;
- thrombocytopenia;
- aplastic anemia (anemia due to the bone marrow’s inability to produce platelets, red and white blood cells);
- lymphadenitis (inflammation of the lymph nodes);
- anaphylaxis including related symptoms of peripheral, angioneurotic and facial emema;
- agitation;
- ocular palsies;
- necrotizing retinitis (inflammation of the eye);
- nerve deafness;
- optic and retrobulbar neuritis (inflammation of the optic nerve);
- Bell’s palsy (sudden but temporary weakness of one half of the face);
- cerebrovascular accident (stroke);
- acute disseminated encephalomyelitis;
- measles inclusion body encephalitis;
- transverse myelitis;
- encephalopathy;
- Guillain-Barré syndrome;
- syncope (fainting);
- tremor;
- dizziness;
- paraesthesia;
- febrile seizure;
- afebrile seizures or convulsions;
- polyneuropathy (dysfunction of numerous peripheral nerves of the body);
- Stevens-Johnson syndrome;
- Henoch-Schönlein purpura;
- acute hemorrhagic edema of infancy;
- erythema multiforme;
- panniculitis;
- arthritis;
- death
A 2014 published study on the MMR-V vaccine in Canada determined that the risk of febrile seizures to be double in children receiving the MMR-V vaccine when compared to those receiving the MMR and varicella vaccines separately.[7]
A 2015 meta-analysis found a two-fold increase in febrile seizures between 5 and 12 days or 7 and 10 days following MMR-V vaccination in children between the ages of 10 and 24 months.[8]
MMR-V vaccine contains albumin, a human blood derivative, and as a result, a theoretical risk of contamination with Creutzfeldt-Jakob disease (CJD) exists.
Merck states that no cases of transmission of CJD or other viral diseases have been identified and virus pools, cells, bovine serum, and human albumin used in vaccine manufacturing are all tested to assure the final product is free of potentially harmful agents.
Serious complications reported by Merck in the MMRII product insert during vaccine post-marketing surveillance include:[9]
- brain inflammation (encephalitis) and encephalopathy (chronic brain dysfunction);
- panniculitis (inflammation of the fat layer under the skin);
- atypical measles;
- syncope (sudden loss of consciousness, fainting);
- vasculitis (inflammation of the blood vessels);
- pancreatitis (inflammation of the pancreas);
- diabetes mellitus;
- thrombocytopenia purpura (blood disorder);
- Henoch-Schönlein purpura (inflammation and bleeding in the small blood vessels);
- acute hemorrhagic edema of infancy (rare vasculitis of the skin’s small vessels occurring in infants);
- leukocytosis (high white blood cell count);
- anaphylaxis (shock);
- bronchial spasms;
- pneumonia;
- pneumonitis(inflammation of the lung tissues);
- arthritis and arthralgia (joint pain);
- myalgia (muscle pain);
- polyneuritis (inflammation of several nerves simultaneously);
- measles inclusion body encephalitis (disease affecting the brain of immunocompromised persons);
- subacute sclerosing panencephalitis (fatal progressive brain disorder caused by exposure to the measles virus);
- Guillain-Barre Syndrome (GBS)(disease where the body’s immune system attacks the nerves);
- acute disseminated encephalomyelitis (ADEM) (brief widespread inflammation of the nerve’s protective covering);
- transverse myelitis (inflammation of the spinal cord);
- aseptic meningitis;
- erythema multiforme (skin disorder from an allergic reaction or infection);
- urticarial rash (hives, itching from an allergic reaction);
- measles-like rash;
- Stevens-Johnson syndrome (severe reaction causing the skin and mucous membranes to blister, die, and shed);
- nerve deafness (hearing loss from damage to the inner ear);
- otitis media (ear infection);
- retinitis (inflammation of the retina of the eye);
- optic neuritis (inflammation of the optic nerve);
- conjunctivitis (pink eye);
- ocular palsies (dysfunction of the ocular nerve);
- epididymitis (inflammation of the epididymis);
- paresthesia (burning or prickling of the skin);
- death.
In the comprehensive report evaluating scientific evidence, Adverse Effects of Vaccines: Evidence and Causality,[10] published in 2012 by the Institute of Medicine (IOM), 30 reported vaccine adverse events following the Measles, Mumps, and Rubella (MMR) vaccine were evaluated by a physician committee.[11]
These adverse events included measles inclusion body encephalitis, febrile seizures, arthritis, meningitis, Guillain Barre Syndrome, autism, diabetes mellitus, optic neuritis, transverse myelitis and more.
In 23 of the 30 measles, mumps, and rubella (MMR) vaccine-related adverse events evaluated, the IOM committee concluded that there was inadequate evidence to support or reject a causal relationship between the MMR vaccine and the reported adverse event, primarily because there was either an absence of methodologically sound published studies or too few quality studies to make a determination.[12]
The IOM committee, however, concluded that the scientific evidence “convincingly supports” a causal relationship between febrile seizures, anaphylaxis, and measles inclusion body encephalitis in immunocompromised individuals and the MMR vaccine and favored acceptance of a causal relationship between transient arthralgia in both children and women and the MMR vaccine.[13]
The IOM committee also concluded that it favored rejection of a causal association between both autism and the MMR vaccine and Type 1 diabetes and the MMR vaccine, however, both of these conclusions were made following the review of only five epidemiological studies.[14]
In 2012, the Cochrane Collaborative examined 57 studies and clinical trials involving approximately 14.7 million children who had received the MMR vaccine.[15]
While the study authors stated that they were not able to detect a “significant” association between MMR vaccine and autism, asthma, leukemia, hay fever, type I diabetes, gait disturbance, Crohn’s disease, demyelinating diseases or bacterial or viral infections, they reported that:
“The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate.”[16]
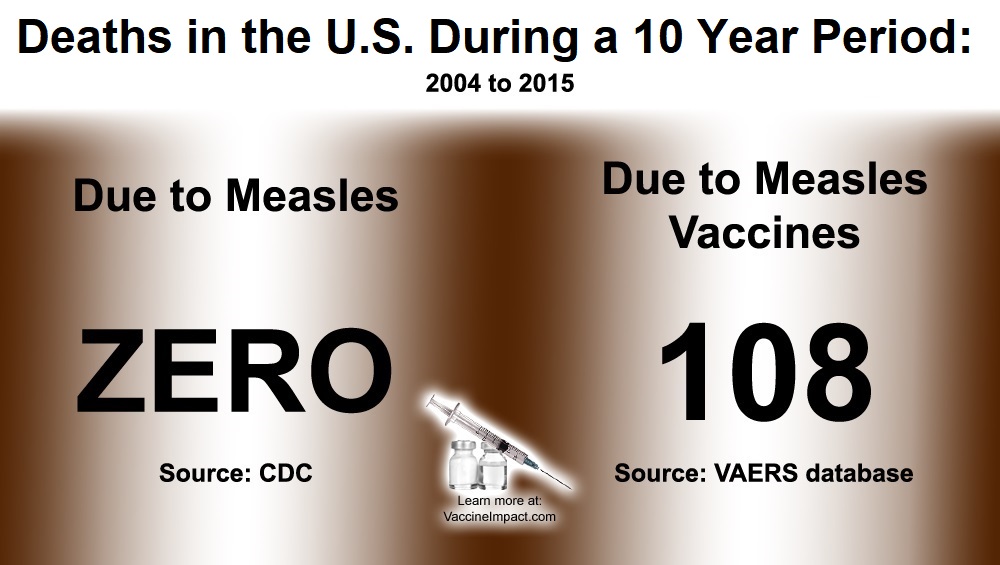
As of November 30, 2018, there have been more than 93,179 reports of measles vaccine reactions, hospitalizations, injuries and deaths following measles vaccinations made to the federal Vaccine Adverse Events Reporting System (VAERS), including 459 related deaths, 6,936 hospitalizations, and 1,748 related disabilities.
Over 50% of those adverse events occurred in children three years old and under. However, the numbers of vaccine-related injuries and deaths reported to VAERS may not reflect the true number of serious health problems that occur develop after MMR vaccination.
Even though the National Childhood Vaccine Injury Act of 1986 legally required pediatricians and other vaccine providers to report serious health problems following vaccination to federal health agencies (VAERS), many doctors and other medical workers giving vaccines to children and adults fail to report vaccine-related health problem to VAERS.
There is evidence that only between one and 10 percent of serious health problems that occur after use of prescription drugs or vaccines in the U.S. are ever reported to federal health officials, who are responsible for regulating the safety of drugs and vaccines and issue national vaccine policy recommendations.[17] [18] [19] [20] [21]
As of January 2, 2019, there have been 1,258 claims filed so far in the federal Vaccine Injury Compensation Program (VICP) for 82 deaths and 1,176 injuries that occurred after measles vaccination.
Of that number, the U.S. Court of Claims administering the VICP has compensated 483 children and adults, who have filed claims for measles vaccine injury.[22]
One example of an MMR vaccine injury claim awarded compensation in the VICP is the case of O.R. On February 13, 2013, O.R. received the MMR, Haemophilus Influenzae type B, Pneumococcal (Prevnar 13), Hepatitis A, and Varicella vaccines.
That evening, following vaccination, she became feverish and irritable prompting her mother to contact the doctor.
The doctor advised O.R.’s mom to administer Benadryl and Tylenol for her symptoms.
The fever persisted for several days and was followed by a severe seizure resulting in cardiac and respiratory arrest. The cardiac arrest and seizures caused O.R. to develop encephalopathy, kidney failure, severe brain injury, low muscle tone and cortical vision impairment.
After several months of inpatient hospitalization, O.R. was discharged home with 24-hour supervised medical care.[23]
On November 20, 2017, the court conceded that the MMR vaccine caused her encephalopathy and O.R. was awarded a $101 million dollar settlement to cover medical expenses for the rest of her life.[24] [25]
In 1998, public health officials and attorneys associated with the federal Vaccine Injury Compensation Program published a review in Pediatrics in regards to the medical records of 48 children ages 10 to 49 months, who received a measles vaccine or combination MMR vaccine between 1970 and 1993 and developed encephalopathy after vaccination.
The children either died or were left with permanent brain dysfunction, including mental regression and retardation, chronic seizures, motor and sensory deficits and movement disorders. The study authors concluded that:
“The onset of neurologic signs or symptoms occurred with a nonrandom, statistically significant distribution of cases on days 8 and 9. No cases were identified after the administration of monovalent mumps or rubella vaccine. This clustering suggests that a causal relationship between measles vaccine and encephalopathy may exist as a rare complication of measles vaccination.”[26]
Nearly two decades earlier, in 1981, a report of the National Childhood Encephalopathy Study was published in Britain that concluded:
“The risk of a serious neurological disorder within 14 days after measles vaccine in previously normal children irrespective of eventual clinical outcome is 1 in 87,000 immunizations.”[27]
However, a 2007 study conducted in Britain concluded “We can estimate the vaccine-attributable risk of serious neurologic disease after the first dose of MMR vaccine as 1 in 365,000 doses.[28]
As well, published studies have shown that the MMR vaccine components or excipients, particularly egg antigens and porcine or bovine gelatin, can trigger both immediate and delayed anaphylactic reactions.[29] [30]
In Guinea-Bissau, Dr. Peter Aaby has studied and administered vaccines to thousands of children for more than three decades and has published research on vaccine safety and effectiveness, including research on measles and measles vaccine.[31]
He found that there can be marked differences in the way that boys and girls respond to vaccines and noted an increased risk of mortality in girls who received DTP and measles vaccines at the same time.[32]
He also found that fatality rates were increased for children ages 6 months to 17 months old, if they had received the DTP vaccine simultaneously with or after receiving measles vaccine.[33]
In 1995, Swiss researchers discovered the presence of the reverse transcriptase (RT) enzyme in the live measles and mumps vaccine, and traced it back to the cells of the chickens used to create the vaccine.[34]
Reverse transcriptase is responsible for copying RNA into DNA and its activity is associated with the presence of retroviruses, a class of viruses that has the ability to permanently alter the genes of the cells they infect.
While the World Health Organization (WHO) and the CDC reviewed the findings, they were also quick to dismiss them, with the CDC publicly stating that “we are not investigating a situation in which there has been any adverse reaction at all.”[35]
Independent researchers have expressed concerns that the use of animal tissues for the production of human vaccines such as the live MMR vaccine may facilitate the transfer of viral infection from animals into man causing as yet undetected and unevaluated negative health effects on humans.[36]
The first evidence of persistent measles virus infection of the intestine after measles vaccination was discovered in 1995 by British researchers.[37]
In 1998, an association between live virus measles vaccine, inflammatory bowel disease (IBD) and regressive autism was hypothesized by gastroenterologist Dr. Andrew Wakefield and other physicians at Royal Free Hospital after they detected the presence of measles virus in the intestines of children suffering with Crohn’s disease and autism.
The paper, published in The Lancet, suggested MMR vaccine may be associated with development of regressive autism in previously healthy children, was immediately met with intense anger and criticism from public health officials and medical trade associations.[38]
Hans Asperger had observed a high rate of gastrointestinal (celiac) disease in children diagnosed with autism,[39] and these observations prompted Wakefield and his colleagues to further examine this association.
After studying children who were suffering from inflammatory bowel disease and were receiving treatment at Royal Free Hospital in the United Kingdom, the researchers hypothesized that a persistent viral infection, either from natural measles disease or live virus measles vaccine, could cause chronic inflammation in the bowel and damage to the central nervous system in some children.
However, in their paper they emphasized that they had not proven a cause and effect relationship between autism, MMR vaccine and non-specific colitis, which they described as “autistic ileal-lymphoid-nodular hyperplasia,” but rather called for more studies to explore the potential relationship.[40]
Additional independent studies on this subject have also reported the presence of measles virus in association with gastrointestinal disorders, such as enterocolitis and chronic intestinal inflammation.[41] [42]
Today, the majority of doctors and health officials reject the suggestion that MMR vaccine is associated with the development of autism in children.[43]
However, privately funded research continues to investigate the potential association between vaccines, including MMR vaccine, and the development of autism, inflammatory bowel disease and other kinds of brain and immune system dysfunction in previously healthy children.
The MMRII and the ProQuad (MMR-V) product inserts report the following:
- Measles inclusion body encephalitis, pneumonitis, and death have occurred in severely immunocompromised individuals who were inadvertently vaccinated. Disseminated mumps and rubella infections have also been reported in this population.
- Subacute sclerosing panencephalitis (SSPE) has been reported in children without a history of wild-type measles infection, however, these children were documented to have received measles vaccine. The vaccine product insert speculates that some cases may have either resulted from measles vaccination or from a possible unrecognized case of measles in the first year of life.
- In the majority of susceptible individuals, small amounts of the live attenuated rubella virus have been excreted from the throat or nose 7 to 28 days following vaccination. According to the vaccine product insert, no evidence has confirmed that the rubella virus can be transmitted to susceptible individuals who come into contact with vaccinated persons. Transmission through close personal contact have been accepted as being theoretically possible but it is not considered a significant risk.
- Transmission of the rubella vaccine virus through breast milk has been noted and postpartum women vaccinated with a live attenuated rubella vaccine may transmit the virus to their breast-fed infants. In one study, several infants were found to have serological evidence of rubella infection without severe disease, however, one infant was noted to have a mild illness found to be typical of rubella.
- Vaccine product inserts for MMR and MMR-V deny any reports of transmission of live attenuated mumps or measles viruses from persons vaccinated and susceptible close contacts. Measles or mumps vaccine virus secretion in human milk is not known.
In November 2014, the National Vaccine Information Center published a special report The Emerging Risks of Live Virus and Virus Vectored Vaccines: Vaccine Strain Virus Infection, Shedding and Transmission.[44]
This report reviewed the medical literature for evidence that live virus vaccine strain infection, shedding, and potential for transmission occurs, including measles vaccine strain infection and shedding.
There have been published reports of vaccine strain measles infection with clinical symptoms that are indistinguishable from wild-type measles.[45] [46]
There are also a few reports of measles vaccine strain virus shedding and lab confirmed infection in children following MMR vaccination. In 2002, there was a published report by researchers in France of
“a child presenting with fever 8 days after vaccination with a measles-mumps-rubella vaccine. Measles virus was isolated in a throat swab taken 4 days after fever onset. This virus was then further genetically characterized as a vaccine-type virus.” [47]
In 2010, Eurosurveillance published a report about the shedding of vaccine strain measles virus in urine and throat secretions of a Croatian child with vaccine-associated rash illness.[48]
A healthy 14-month old child was given MMR vaccine and eight days later developed macular rash and fever. Lab testing of throat and urine samples between two and four weeks after vaccination tested positive for vaccine strain measles virus. Authors of the report pointed out that when children experience a fever and rash after MMR vaccination, only molecular lab testing can determine whether the symptoms are due to vaccine strain measles virus infection. They stated:
“According to WHO guidelines for measles and rubella elimination, routine discrimination between aetiologies of febrile rash disease is done by virus detection.
However, in a patient recently MMR-vaccinated, only molecular techniques can differentiate between wild type measles or rubella infection or vaccine-associated disease. This case report demonstrates that excretion of Schwartz measles virus occurs in vaccinees.”[49]
In 2012, a report was also published describing a healthy 15-month old child in Canada, who developed irritability, fever, cough, conjunctivitis and rash within seven days of an MMR shot.[50]
Blood, urine and throat swab tests confirmed a vaccine strain measles virus infection 12 days after vaccination. Addressing the potential for measles vaccine strain virus transmission to others, the authors stated,
“While the attenuated virus can be detected in clinical specimens following immunization, it is understood that administration of the MMR vaccine to immunocompetent individuals does not carry the risk of secondary transmission to susceptible hosts.”
IMPORTANT NOTE: Even though ACIP says it’s safe to give other viral and bacterial vaccines at the same time as MMR vaccine, Merck’s MMRII product information insert states that other live virus vaccines—such as varicella[51] should NOT be given at the same time as MMR vaccine but rather should be administered one month prior or one month after MMR vaccination.[52]
Read the full report at NVIC.org.
References
[1] CDC MMR (Measles, Mumps, & Rubella) VIS Feb. 12, 2018
[2] CDC MMRV (Measles, Mumps, Rubella & Varicella) VIS Feb. 12, 2018
[3] CDC Use of Combination Measles, Mumps, Rubella, and Varicella Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. May 7, 2010; 59(RR03);1-12
[4] CDC MMR (Measles, Mumps, & Rubella) VIS Feb. 12, 2018
[5] CDC MMRV (Measles, Mumps, Rubella & Varicella) VIS Feb. 12, 2018
[6] FDA Measles, Mumps, Rubella and Varicella Virus Vaccine Live. Oct 23, 2018
[7] MacDonald SE, Dover DC, Simmonds KA, et al. Risk of febrile seizures after first dose of measles–mumps–rubella–varicella vaccine: a population-based cohort study. CMAJ. 2014 Aug 5; 186(11): 824–829.
[8] Ma SJ, Xiong YQ, Jiang LN et al. Risk of febrile seizure after measles-mumps-rubella-varicella vaccine: A systematic review and meta-analysis. Vaccine. 2015 Jul 17;33(31):3636-49
[9] FDA Measles, Mumps and Rubella Virus Vaccine, Live May 16, 2017
[10] Institute of Medicine Committee to Review Adverse Effects of Vaccines. Adverse Effects of Vaccines: Evidence and Causality.(Evaluating Biological Mechanisms for Adverse Events: Increased Susceptibility). Washington, DC: The National Academies Press. 2012
[11] Institute of Medicine Committee to Review Adverse Effects of Vaccines. Adverse Effects of Vaccines: Evidence and Causality.(Evaluating Biological Mechanisms for Adverse Events: Increased Susceptibility). Washington, DC: The National Academies Press. 2012. Chap. 4 (103-238)
[12] Ibid
[13] Ibid
[14] Ibid
[15] Demicheli V, Rivetti A, et al. (Intervention Review) Vaccines for Measles, Mumps and Rubella in Children. The Cochrane Library 2012, Issue 2.
[16] Ibid
[17] Kessler DA, the Working Group, Natanblut S, et al. A New Approach to Reporting Medication and Device Adverse Effects and Product Problems. JAMA. 1993;269(21):2765-2768.
[18] FDA.gov. Kessler DA. Introducing MEDWatch: A New Approach to Reporting Medication and Device Adverse Effects and Product Problems. Reprint from JAMA. June 9, 1993.
[19] Braun M. Vaccine adverse event reporting system (VAERS): usefulness and limitations. Johns Hopkins Bloomberg School of Public Health
[20] Rosenthanl S, Chen R. The reporting sensitivities of two passive surveillance systems for vaccine adverse events. Am J Public Health 1995; 85: pp. 1706-9.
[21] AHRQ Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS) Dec 1, 2007-Sep. 30, 2010
[22] U.S. Department of Health and Human Services. National Vaccine Injury Compensation Program Data—January 2, 2019.National Vaccine Injury Compensation Program. Jan. 2, 2019
[23] MCTlawyers.com $101 Million Dollar Vaccine Injury Award for Encephalopathy from MMR Vaccine. Press Release. Jul. 17, 2018
[24] Ibid
[25] Office of Special Masters. United States Court of Federal Claims. RAYMOND ROACH, on behalf of O.G.R., a minor child V. Secretary of Health and Human Services. Nov. 20, 2017
[26] Weibel RE, Casserta V, Benor DE et al. Acute Encephalopathy Followed by Permanent Brain Injury or Death Associated with Further Attenuated Measles Vaccine: A Review of Claims Submitted to the National Vaccine Injury Compensation Program.Pediatrics 1998; 101(3): 383-387.
[27] Alderslade R, Bellman MH, Rawson NSB, Ross EM, Miller DL. The National Childhood Encephalopathy Study: A Report on 1000 Cases of Serious Neurological Disorders in Infants and Young Children from the NCES Research Team. Her Majesty’s Stationery Office 1981.
[28] Ward KN, Bryan NJ, Andrew NJ et al. Risk of Serious Neurologic Disease After Immunization of Young Children in Britain and Ireland. Pediatrics 2007; 120(2): 314-321.
[29] Lakshman R. MMR Vaccine and Allergy. Arch Dis Child 2000;82:93-95.
[30] Bogdanovic, J, Halsey NA, Wood RA, et al. Bovine and Porcine Gelatin Sensitivity in Milk and Meat-Sensitized Children J Allergy Clin Immunol. 2009 Nov; 124(5): 1108–1110.
[31] Bandim Health Project Staff – Peter Aaby Oct. 6, 2017
[32] Aaby P, Jensen H, Samb B, et al. Differences in Female-Male Mortality after High-Titre Measles Vaccine and Association with Subsequent Vaccination with Diphtheria-Tetanus-Pertussis and Inactivated Poliovirus: Reanalysis of West African Studies. Lancet. 2003;361:2183-8
[33] Aaby P, Biai S, Veirum JE, et al. DTP with or after Measles Vaccination Is Associated with Increased In-Hospital Mortality in Guinea-Bissau. Vaccine. Jan. 26, 2007 Jan 26; (25)7: 1265-1269.
[34] Böni J, Stalder J, Reigel F et al. Detectionof reverse transcriptase activity in live attenuated virus vaccines. Clin Diagn Virol.1996 Feb;5(1):43-53.
[35] Brown D UNEXPECTED PROTEIN FOUND IN MEASLES-MUMPS VACCINE. The Washington Post. Dec. 9, 1995
[36] Fisher B Virus Enzyme Found in MMR Vaccine. The Vaccine Reaction. Nov. 28, 2018
[37] Lewin J, Dhillon AP, Sim R et al. Persistent measles virus infection of the intestine: confirmation by immunogold electron microscopy. Gut. 1995 Apr; 36(4):564-9.
[38] NVIC. Research Into Vaccines, Autism and Intestinal Disorders Published in The Lancet. Press Release: March 3, 1998.
[39] Brown A, Chow D, Murakami S, et al. Possible gastrointestinal symptoms in a subset of children with autism Expert Rev Gastroenterol Hepatol 2010 4(2), 125–127
[40] Wakefield AJ, Murch SH, Anthony A, et al Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998 Feb 28; 351(9103):637-41.(Retracted)
[41] Ashwood P, Anthony A, Pellicer AA et al. Intestinal Lymphocyte Populations in Children with Regressive Autism: Evidence for Extensive Mucosal Immunopathology J Clin Immunol. 2003 Nov; 23(6):504-17.
[42] Mercola J New Evidence Refutes Fraud Findings in Dr. Wakefield Case Jan. 24, 2012
[43] CDC Measles, Mumps, and Rubella (MMR) Vaccine Safety Aug 21, 2018
[44] Fisher BL. The Emerging Risks of Live Virus and Virus Vectored Vaccines: Vaccine Strain Virus Infection, Shedding and Transmission. NVIC November 2014.
[45] Jenkins GA, Chibo D, Kelly HA et al. What is the cause of a rash after measles-mumps-rubella vaccination? Med J Aust 1999; 171(4): 194-195.
[46] Berggren KL, Tharp M, Boyer KM. Vaccine-associated “wild-type” measles. Pediatr Dermatol 2005; 22(2): 130-132.
[47] Morfin F, Beguin A, Lina B, et al. Detection of measles vaccine in the throat of a vaccinated child. Vaccine 2002; 20(11-12); 1541-1543.
[48] Kaic B, Gjenero-Margan I, Aleraj B et al. Spotlight on Measles 2010: Excretion of Vaccine Strain Measles Virus in Urine and Pharyngeal Secretions of a Child with Vaccine Associated Febrile Rash Illness, Croatia, March 2010. Eurosurveillance 2010 15(35).
[49] Ibid
[50] Nestibo L, Lee BE, Fonesca K et al. Differentiating the wild from the attenuated during a measles outbreak. Paediatr Child Health Apr. 2012; 17(4).
[51] FDA Varivax Oct. 19, 2018
[52] FDA Measles, Mumps and Rubella Virus Vaccine, Live May 16, 2017
Leaving a lucrative career as a nephrologist (kidney doctor), Dr. Suzanne Humphries is now free to actually help cure people.
In this autobiography she explains why good doctors are constrained within the current corrupt medical system from practicing real, ethical medicine.
One of the sane voices when it comes to examining the science behind modern-day vaccines, no pro-vaccine extremist doctors have ever dared to debate her in public.
-
Book – The Vaccine Court, by Wayne Rohde – 240 pages
“The Dark Truth of America’s Vaccine Injury Compensation Program”
FREE Shipping Available!
ORDER HERE!







3 Comments