Comments by John P. Thomas
Health Impact News
HPV vaccines are beginning to be recognized as one of the great medical mistakes of the twenty-first century. The harm caused by these vaccines to girls and now boys throughout the world is staggering.
In the following article written by medical researcher Cynthia Janak, she explains how a large number of deficiencies in the clinical trials for Gardasil® may have prevented the detection of injuries caused by the vaccine.
Specifically, she will look at Gardasil®, manufactured by the pharmaceutical company known as Merck. She will show how research studies allegedly did not collect data that would have revealed injury. She will show how the results from these incomplete research studies were allegedly used to convince the FDA, doctors, and parents that Gardasil® was safe, when in fact true safety was allegedly never determined.
Cynthia Janak has been researching HPV vaccines for the past ten years. At first she wanted to know why former Governor Rick Perry of Texas wanted to mandate this vaccine. As she researched this, she soon became aware of illnesses caused by HPV vaccination. This led her to an investigation of how HPV vaccines harm young people, and how health can be restored.
This article is her latest work on the HPV vaccine, which is causing disability, infertility, and even death in young people. As you will see from the resources she quotes, Merck continues to sell Gardasil® as if there was never a documented case of harm.
Now let’s take a look at the overwhelming collection of evidence that Cynthia Janak has assembled.
Gardasil Trials
by Cynthia A. Janak
Health Impact News
My Goal in Writing this Article
The key question that must be asked is this: Did Merck intentionally deceive the FDA, doctors, and parents with its Gardasil® vaccine research? In other words, did Merck design rat studies and human clinical studies that would fail to identify vaccine harm, and give a false sense of security to those who would approve or use the vaccine?
Based on the evidence I have uncovered, I believe it is possible to conclude that its future marketing plans and the protection of Merck’s profitability for its shareholders were higher priorities than the safety of the HPV vaccine called Gardasil®.
What is Merck’s Top Priority?
Please keep this statement from Merck in mind as you read about the science that I will discuss. This is what the Merck website tells us:
The primary mission of our Board is to represent and protect the long-term interests of the company’s shareholders. [7]
First Fundamental Question
Does Gardasil® Produce Endocrine Disruption?
My latest research on the Gardasil® vaccine has been to see if it causes endocrine disruption. This led me to an excellent article published in January of 2016 by the American College of Pediatricians (ACP). [1] The ACP voiced concerns about premature ovarian failure (POF) or premature menopause, which can occur after vaccination with Gardasil®. They did such a great job that I am going to quote their concerns.
The article is written as a warning to pediatricians about HPV vaccines. It tells them, “There are legitimate concerns that should be addressed.” These are the concerns they stated:
(1) Long-term ovarian function was not assessed in either the original rat safety studies, or in the human vaccine trials,
(2) Most primary care physicians are probably unaware of a possible association between HPV4 and POF and may not consider reporting POF cases or prolonged amenorrhea (missing menstrual periods) to the Vaccine Adverse Event Reporting System (VAERS),
(3) Potential mechanisms of action have been postulated based on autoimmune associations with the aluminum adjuvant used and previously documented ovarian toxicity in rats from another component, polysorbate 80,
(4) since licensure of Gardasil® in 2006, there have been about 213 VAERS reports (per the publicly available CDC WONDER VAERS database) involving amenorrhea, POF or premature menopause, 88% of which have been associated with Gardasil®. The two-strain HPV2, Cervarix™, was licensed late in 2009 and accounts for 4.7% of VAERS amenorrhea reports since 2006, and 8.5% of those reports from February 2010 through May 2015. This compares to the pre-HPV vaccine period from 1990 to 2006 during which no cases of POF or premature menopause and 32 cases of amenorrhea were reported to VAERS. [1]
There were NO cases of POF or premature menopause reported prior to 2006. Since Gardasil ® and Cervarix™ were approved there have been 198 cases of amenorrhea (absence of menstruation) reported which is an 83.3% increase. This number is probably only 1% of the total cases because of under-reporting. According to David Kessler, former commissioner of the FDA, “Only about one percent of serious events are reported. Less serious vaccine adverse events (e.g., swelling, fever, or redness at the vaccination site) are more under-reported than more serious vaccine adverse events (e.g., hospitalizations and death).” [10] Further in my article I will explain why under-reporting is not only possible but probable.
More Questions
The ACP report raised several questions that I wanted to answer: What is so important about rat studies? What are the details of the case studies that were mentioned in the ACP article? How many cases of POF, amenorrhea or associated conditions have been reported to VAERS (Vaccine Adverse Event Reporting System)?
I will answer these questions in the following sections and will present detailed data from the VAERS database near the end of this article.
Could the Research Design Measure Ovarian Dysfunction?
The American College of Pediatricians stated:
Few other vaccines besides Gardasil® that are administered in adolescence contain polysorbate 80. Pre-licensure safety trials for Gardasil® used placebo that contained polysorbate 80 as well as aluminum adjuvant. Therefore, if such ingredients could cause ovarian dysfunction, an increase in amenorrhea probably would not have been detected in the placebo controlled trials. Furthermore, a large number of girls in the original trials were taking hormonal contraceptives which can mask ovarian dysfunction including amenorrhea and ovarian failure. Thus a causal relationship between human papillomavirus vaccines (if not Gardasil® specifically) and ovarian dysfunction cannot be ruled out at this time.
While data from those studies do not indicate an increased rate of amenorrhea after vaccination, the essential lack of saline placebos and the majority of participants taking hormonal contraceptives in those studies preclude meaningful data to rule out an effect on ovarian function. [1]
These statements by the ACP are very powerful. They tell us about some of the outcomes from this vaccine with regards to fertility and safety. These physicians seem very concerned that the studies did not provide meaningful data that could rule out an effect on ovarian function.
Ovarian Insufficiency Following HPV Vaccination
Australian researchers put together a case study of three young women (ages 16, 16, and 18) who experienced premature ovarian insufficiency after receiving HPV vaccine. This study is not difficult to read, and I recommend that everyone read it.
See: Adolescent Premature Ovarian Insufficiency Following Human Papillomavirus Vaccination
In this article, the researchers stated:
Vaccine research does not present an ovary histology report of tested rats but does present a testicular histology report. Enduring ovarian capacity and duration of function following vaccination is unresearched in preclinical studies, clinical and postlicensure studies. Postmarketing surveillance does not accurately represent diagnoses in adverse event notifications and can neither represent unnotified cases nor compare incident statistics with vaccine course administration rates. The potential significance of a case series of adolescents with idiopathic premature ovarian insufficiency following HPV vaccination presenting to a general practice warrants further research. Preservation of reproductive health is a primary concern in the recipient target group. Since this group includes all prepubertal and pubertal young women, demonstration of ongoing, uncompromised safety for the ovary is urgently required. This matter needs to be resolved for the purposes of population health and public vaccine confidence. [2]
What is interesting here is that sufficient studies were done for male rats but not for female rats. At least that is what we are led to believe by Merck’s incomplete study data.
Animal Studies are Required to Prove Safety
Animal studies are required prior to the approval of any medication or vaccine for human trials. The animal studies should be designed to identify safety issues that might come up. Merck did animal studies; but they appear to have omitted specific testing to measure the effects of their vaccine on female reproduction.
Merck Studies did not Examine Rat Ovaries
This is what the Australian research scientists learned when they tried to find out more information about the rat studies that were used to “prove” the safety of HPV vaccine. They stated:
No histology report of the vaccine-tested rodent ovary was available under Freedom of Information Request to the Therapeutic Goods Administration of Australia. There is no cellular observation available on tested rodents’ ovaries beyond a numbering of corpora lutea present on the ovary at caesarian section. [2]
What does Merck’s lack of full examination of female rats communicate?
Merck did not Give Three Doses of HPV Vaccine to the Rats
These rats only received 2 doses of the HPV vaccine, when 3 doses are required for humans. Why?
The Australian researchers stated:
In preclinical fertility studies submitted at licensing, no rats were tested with the complete vaccination course, with representative interval administration, prior to mating. The study concludes that vaccine rodent fertility testing conferred ‘a safety margin of 200-fold by body weight for adolescents.’ ‘Guidance for Industry’ research guidelines state ‘where possible we recommend that you administer the maximum human dose (eg, 1 human dose = 1 rabbit dose) regardless of body weight.’ The reason for omission of the third vaccination dose prior to measuring the rats’ capacity to conceive is unclear. [2]
Yes, it is unclear. Could it be that Merck would not want proof that the vaccine is unsafe? They need to protect their marketing plans and the interests of the shareholders with this novel vaccine.
Australian researchers further stated:
The 200-fold safety prediction was derived from the 0.25 kg (0.55 lbs.) weight of a rat compared with the “average body weight of an adolescent girl (50 kg [110.2 lbs.]).” The HPV4 target girl group is aged from 9 years and administration in Australia is to girls aged 12 and 13 years under the National Immunization Programme. The 50th centile weight of 9-year-olds is 28 kg (61.7 lbs.), of 12-year-olds is 42 kg (92.5 lbs.), and of 13-year-olds is 46 kg (101.4 lbs.). Australian age-specific weights therefore also reduce modeled calculations of fertility safety. [2]
Here in the United States, we start administering HPV injections at the age of 11. Thus, we see that the rat studies did not address fertility safety for the right age group of girls in Australia or in the United States.
Merck did not Look at Long-term Fertility
The researchers from Australia stated:
Long-term fecundity (fertility) studies of vaccinated female rodents’ duration of reproductive lifespan, recorded numbers of litters and pup numbers in subsequent litters were also requested under the original freedom of information application but were unavailable. [2]
Did Merck deliberately withhold vital information about future fertility of the litters? Could it be that this information would have damaged Merck’s Gardasil® application? We just don’t know for sure, but it causes one to wonder why the information was not available.
Merck did not Fully Examine Ovarian Function in Girls
Even if ovarian function in rats was overlooked, we would expect that Merck researchers would pay close attention to fertility in girls when they did their clinical trials. Unfortunately, this was not the case.
The Australian scientists further explain what happened. They stated:
Research consideration of ongoing female fertility was similarly absent from phases II and III clinical safety studies. The capacity of safety studies to assess ovarian function, particularly of the target age group, was reduced by several factors. [2]
In protocol 016, 240 girls (aged 10-15 years) were left in the study at 12 months, comprising 47.4% of screened healthy participant younger girls. Immune response data were collected through month 7, and safety data through to month 12. More than 52% were lost from the 12-month safety follow-up instituted as a protocol amendment. Loss of the majority of participants to safety observation significantly compromised this trial as a safety study of younger adolescents forming the vaccine’s target group. … [2]
Protocol 018 fully vaccinated 587 girls. A total of 52.3% of enrolled girls were aged 9 to 12 years. It is not clear what proportions of girls in these target group safety studies could potentially have reported menstrual cycle patterns or aberrations of patterns. Similarly, health interviews with the participants 18 months after the first vaccination may not have been able to determine menstrual abnormalities while cycles are commencing or establishing ovulatory patterns. [2]
Let’s look at protocol 018. We do not know how many girls had started their menstruation, had regular cycles or none at all prior to the study. What better way is there to hide infertility as a side effect than by injecting someone who has not yet established their ovulatory pattern?
Merck Encouraged Girls to use Birth Control Pills
As we continue our journey, we now find a new revelation. Australian researchers explain how birth control pills affected the research. They stated:
Given the masking effects of hormonal contraception on ovarian function it is relevant that contraceptive hormone usage was reported at 58% to 60% of vaccine recipients in safety trials at baseline interview in phase III studies. This rose to 68% to 83% of participants in the 2 substudies of protocol 013.42(p143) In all, 75% to 82% used hormonal contraception within 15 days of any vaccination in trial 007,42(p216) and more than two thirds recorded concomitant hormonal contraception usage within 14 days of any vaccination in protocol 015. (p244) Phase III studies’ participants, mostly 16 years and older, were required to use effective contraception for at least 7 months.
A major review of the HPV4 vaccine safety profile reports: ‘new medical conditions were not considered adverse events if they occurred post month 7, or were not considered by the investigator to be vaccine related.’ [2]
I want to emphasize these last points. Up to 82% of subjects used hormonal contraception, and in Phase III studies participants mostly over the age of 16 were required to use contraception for at least 7 months.
If study participants reported a “new medical condition” after month 7, then it would not even be documented as an adverse event related to the vaccine. Why? Because the study protocols only record adverse events to month seven. [8] This may have altered the data in favor of the vaccine.
Australian researchers further emphasize their concerns about the use of hormonal contraceptives in the studies. They stated:
We all know that hormonal contraceptives help some females have regular menses. What we did not know is that their usage during the studies could skew the safety data. [2]
Merck didn’t Properly Measure Menstrual Activity after Vaccination
To determine whether a vaccine affected menstrual cycles, it is necessary to collect data about menstrual activity over a long period of time. The Australian scientists explain the shortcomings in the human clinical trials. They stated:
The design of safety studies with use of a vaccine report card further restricted the recording and reporting of menstrual dysfunction. The largest safety study, phase III study protocol 015, enrolled older females predominantly aged 16 to 23 years (1 was 15 years old, 46 were older than 23 years) of whom 5916 completed the 3-dose HPV4 vaccination period and 5953 completed placebo dosages. (p58) [2]
A subgroup selected from among these formed the Detailed Safety Cohort. It followed 448 recipients of at least 1 vaccination and 447 control recipients, … However, menstrual cycle disruption, oligomenorrhea, and amenorrhea will not signal as SAEs by definition. SAEs are defined as life-threatening, resulting in death, permanent disability, congenital anomaly, hospitalization, prolongation of hospitalization, or necessitating medical or surgical intervention to prevent one of these outcomes. [2]
The use of a vaccine report card to record other adverse events occurring within 2 weeks of each vaccination has limited ability to detect diminishing menstrual cycles. This is a weakness in the safety design of clinical trials. It would not have detected the menstrual cycle decline evident in the cases of premature ovarian insufficiency presented in this series. Protocol 018 VRC prompted for additional information such as headaches, rashes, muscle/joint pain, and diarrhea that occurred within 14 days but not menstrual aberration. [2]
It’s important to clearly understand what is being said here.
Only a select group of individuals (448 out of 5916) were chosen for follow-up. Of these, only one girl age 15 was close to the target age group out of 5916. It would be interesting to know what the criteria were when they chose those individuals.
It also says that menstrual cycle disruption will not be flagged as a serious adverse event (SAE) because of the finite definition of an SAE. They used a definition that would exclude collecting relevant data about disruptions in menstrual cycles.
There is one other problem. The cycle of a female can be between 28 and 32 days. The study only recorded events up to 14 days. So, if there was any menstrual dysfunction it would not be reported anyway. It could take 3 to 6 months to realize problems with a female’s menstrual cycle. Also, the majority were on contraceptives, which would tend to normalize the timing of the cycles.
Merck did not Collect Information about New Medical Conditions
As I mentioned, new medical conditions that developed after vaccination were not collected. Australian researchers explained what happened. They stated:
When the Center for Biologics Evaluation and Research requested an analysis of autoimmune conditions over the entire safety database, the sponsor noted that ‘there were subjects with additional new medical conditions that were not reported in the Clinical Study Reports for 011 and 012 [within protocol 013]. These included two subjects with amenorrhea.’ (p198) [2]
What about the other “new medical conditions” that were also not reported? I would like to know what they were. This could mean that the 73.3% of adverse events recorded under “New Medical Conditions” in the FDA September 11, 2009 report [8] was substantially under-reported.
We will never know whether one, two or three months after the time of the final injection there was some type of menstrual disruption. Without this information, how could Merck declare that their vaccine was safe, and be confident that it would not harm the reproductive systems of young women?
Key Points about Merck’s HPV Safety Study
The two scientists from Australia summarized how Merck compromised safety studies and observation of ovarian health. I will end this section with another quote from their article. They stated:
Underrepresentation of the vaccine’s target age group, incomplete and short-term follow-up, definitional limitations, hormone usage, fortnight restrictions of vaccine report card documentation and the decision not to report new medical conditions as adverse events which occurred post month seven from first vaccination compromised safety studies’ observation of ovarian health. [2]
Why did Merck make these kinds of choices?
In the CDC’s Morbidity and Mortality Weekly Report of July 25th, 2014 [6] they polled only 1% of females and males. You find that there is a difference for girls and boys that received one injection of Gardasil® compared to three injections. For example in girls during 2011, 5,955 received one dose and only 910 received all three. This is a discrepancy of 5,045 that did not complete the series. The boys are the same with 1,023 having received one dose and only one completed the series.
Why didn’t the girls and boys complete the full series of vaccines? Why isn’t the CDC researching this to find out why they never completed the series?
As mentioned in previous sections, Merck also did not give all three doses of the vaccine to rats. Why did Merck do this? Does the FDA know that the full three injections were never used in the rat safety study?
Detergent in Vaccines has Serious Health Effects
There are many substances in vaccines in addition to the ones that are supposed to provide immunity. I have reported about polysorbate 80 (a chemical detergent) in vaccines and the effects that it can have on human health. In my article “HPV vaccine cocktail targets not only HPV” [3] I evaluated the addition of polysorbate 80 to the vaccine solution. Polysorbate 80 has been associated with unusually low blood pressure, heart disease, cancer, reduced growth rates, and reduced postnatal survival.
In that article I noted that polysorbate 80 could very well be the reason for the drop in blood pressure that is being experienced in young people. The National Toxicology Program of the Department of Health and Human Services has information about Toxicity Effects for polysorbate 80 (also called Tween 80). I am adding these key points here, because they directly address the dangers associated with HPV vaccines.
The Toxicology report stated:
LABORATORY ANIMALS: Acute Exposure: Commercial IV amiodarone and polysorbate 80 caused a 60% drop in mean blood pressure and left ventricular maximum dP/dT for at least 30 minutes in dogs… polysorbate 80 is not an inert substance, but is a potent cardiac depressant.
LABORATORY ANIMALS: Acute Exposure: Tween 80 (2.4 ug/mL perfusion fluid) showed a coronary vasodilatory effect and increased the cardiac output in isolated guinea pig and rabbit hearts.
LABORATORY ANIMALS: Chronic Exposure or Carcinogenicity: Whereas only 10% of the rats given IV injections of 106 Walker tumor cells developed metastatic tumors, animals given, with the 1 mL cell suspension, Tween 80 (1%) showed 40% incidence of metastasis.
LABORATORY ANIMALS: Chronic Exposure or Carcinogenicity: /In/ 2 yr feed studies, there was equivocal evidence of /carcinogenicity/ polysorbate 80 in male F344/N rats. [9]
New Information about Polysorbate 80
A new piece of information came to my attention while I was writing this article. Researchers stated:
Polysorbate 80 given intravenously caused a slight blood pressure decrease in dogs, cats, rabbits, and monkeys; decreased blood pressure was not seen in these species after oral administration (Krantz et al, 1951). Perfusion of polysorbate 80 into isolated rabbit or guinea pig hearts led to dilation of coronary vessels and increased cardiac output (Correia da Silva and Paiva, 1970). Polysorbate 80 (10 mg/kg) injected intravenously in human patients produced an increase in cardiac output and a decrease in peripheral vascular resistance (CWA, 1984). Polysorbates have been reported to be histamine releasers (Yamasaki et al, 1969). Intravenous injection of 10 mg/kg polysorbate 80 into dogs produced a marked increase in plasma histamine levels and a severe hypotension that could be prevented by treatment with antihistamines (Masini et aL, 1985); in the same study, it was found that polysorbate 80 released histamine from rat peritoneal mast cells in vitro.
There was some reduction in growth rate, postnatal survival of pups, lactation and breeding efficiency, and longevity in rats fed the diet containing 20% polysorbate 80. [4]
Polysorbates Release a Systemic Histamine Storm in Vaccine-Injured People
Polysorbates have been reported to be histamine releasers. They also produce a marked increase in plasma histamine levels and severe hypotension. This substantiates what I wrote in my earlier article, and it adds a new element. This shows a mechanism for the histamine intolerance now becoming epidemic in the HPV vaccine-injured population. [5] When Polysorbate 80 is combined with L-histidine (the precursor of histamine), then there is the real probability of having a systemic histamine storm.
For years I have tried to find just one animal study with Polysorbate and L-histidine as the primary sources used to create an immune response. Alas, I have not been able to find a single one. If Merck did such a study, then it must not have been published.
Polysorbate 80 Damages Ovaries
Even though Merck didn’t evaluate the effects of HPV vaccine on rat ovaries, other scientists have taken up the research. They have studied Polysorbate 80, which is in Gardasil®, and found that it damages the female reproductive system in rats.
These are some of the research findings, which point to Polysorbate 80 as being a highly probable factor in female vaccine injuries such as those occurring after vaccination with Gardasil®.
Researchers stated:
When polysorbate 80 (‘Tween 80’) was injected into newborn rats, it caused similar ovarian damage to injected diethylstilboestrol. Rat ovary effects occurred at all doses tested over a tenfold range. Since this study provides a relevant ovary histology report of a substance in HPV4 it bears detailed consideration. 1%, 5%, or 10% solutions of polysorbate 80 at 0.1 mL per rat were injected into rats at 4, 5, 6, and 7 days after birth. The oestrous cycle was examined at weeks 10, 14, and 18 of age. Findings were compared with control rats given no treatment; negative controls given water injections; and a ‘positive’ control group given a formulation of 50 µg diethylstilboestrol. Rats injected with polysorbate 80 had an oestrous cycle ranging from 9 to 14 days, compared with 4.3 days average length throughout the test in untreated controls and 9.4 days in diethylstilboestrol injected rats. Postmortem conducted at 20 weeks of age on ‘Tween’/polysorbate 80 tested rats reported:
- All Tween-treated groups showed a statistically significant (P < .001) decrease in the relative weight of the ovaries in comparison with the untreated control. The relative weight (% of body weight) was slightly lower in the 1% Tween 80–treated groups than in the 10% Tween 80–treated groups.
- In the group of 6 rats given the lowest dose of Tween 80 ‘in two rats the uterus was enlarged and had a marked vascular pattern.’
- The 5 rats given diethylstilboestrol showed ‘microscopically degenerated follicles in the ovaries with complete absence of corpora lutea. Findings in the ovaries similar to those in the positive control [diethylstilbestrol control] group were also observed in all of the groups given Tween 80.’
- Abnormal histological findings in the cells lining the uterus were observed in all 17 rats given Tween 80 and resembled the abnormal histology observed in diethylstilboestrol-treated rats, which had high cylindrical epithelial cells and some mitoses. The study concluded, ‘4-day administration of Tween 80 to female rats during the period crucial for the development and function of reproductive organs accelerates the maturation of these organs.’ As well as a prolonged oestrous cycle (reproductive cycle) researchers also noted induction of persistent vaginal oestrous. This was slightly more marked in the 1% solution of Tween 80 than in the 5% or 10% solutions. Statistically significant increased weight of the adrenals (P ≤ .05) was also noted in the 1% polysorbate injected rats.
Histologically evident toxic ovarian effects of polysorbate 80 evidenced 5 months after serial injections into very young rats have not been compared with the histological effect of the HPV4 vaccine course containing 150-µg dosage. The relevance of polysorbate 80 ovarian damage to the cases presented here is unresearched and unknown and assurances of “no biologically plausible” link between HPV4 vaccine and ovarian effects cannot be given.
The role of the aluminum adjuvant as a safety study placebo also requires consideration. The development of an ‘autoimmune/inflammatory syndrome induced by adjuvants’ (ASIA) has been postulated by some immunologists to be implicated in the development of premature ovarian failure. [2]
How Many Girls and Young Women have been Damaged by HPV Vaccines?
Let’s take a look at the numbers.
These tables reflect the symptoms or conditions that were reported to VAERS (Vaccine Adverse Event Reporting System). It is well known that maybe only 1% of vaccine reactions are reported, which is why I inserted the Approx. 100% in these tables.
Reported Symptom – Reports 1% = Approx. 100% =
- Amenorrhoea – 198 – 19,800
- Dysmenorrhoea – 120 – 12,000
- Menopause – 1 – 100
- Menstruation irregular – 215 – 21,500
- Premature menopause – 25 – 2,500
- Total – 559 – 55,900
- Amenorrhoea – Absence of menstruation.
- Dysmenorrhoea – Painful menstruation.
- Menstruation irregular – The majority of the reports state more frequent periods, average is 2 to 2 ½ weeks apart. Also stated are heavier bleeding, intense cramping and changes in color (darker almost black).
Reported Symptom – Reports 1% = Approx. 100% =
- Ovarian abscess – 1 – 100
- Ovarian atrophy – 1 – 100
- Ovarian cancer – 5 – 500
- Ovarian cancer stage III – 1 – 100
- Ovarian cyst – 80 – 8,000
- Ovarian cyst ruptured – 10 – 1,000
- Ovarian cystectomy – 1 – 100
- Ovarian disorder – 9 – 900
- Ovarian enlargement – 3 – 300
- Ovarian failure – 10 – 1,000
- Ovarian germ cell teratoma benign – 1 – 100
- Ovarian granulosa cell tumour – 1 – 100
- Ovarian haemorrhage – 2 – 200
- Ovarian infection – 1 – 100
- Ovarian mass – 2 – 200
- Ovarian operation – 2 – 200
- Ovarian necrosis – 1 – 100
- Ovarian torsion – 1 – 100
- Haemorrhagic ovarian cyst – 4 – 400
- Metastases to ovary – 1 – 100
- Polycystic ovaries- 23 – 2,300
- Total – 160 – 16,000
Reported Symptom – Reports 1% = Approx. 100% =
- Adenocarcinoma of the cervix – 5 – 500
- Biopsy cervix abnormal – 53 – 5,300
- Cervical Dysplasia – early state cancer – 271 – 27,100
- Cervix carcinoma – 53 – 5,300
- Cervix carcinoma stage 0 – 31 – 3,100
- Cervix carcinoma stage I – 3 – 300
- Cervix carcinoma stage II – 2 – 200
- Cervix carcinoma stage III – 1 – 100
- Cervix carcinoma stage IV – 2 -200
- Cervix disorder – 20 – 2,000
- Cervix dystocia – 1 – 100
- Cervix haemorrhage uterine – 1 – 100
- Cervix inflammation – 2 – 200
- Cervix neoplasm – 2 – 200
- Cervix oedema – 1 – 100
- Cervix operation – 1 – 100
- Uterine cervix ulcer – 1 – 100
- Total – 450 – 45,000
Vaccines containing Polysorbates
Vaccine – Manufacturer’s P.I. – Recipient Age – Type
- DTaP (Infanrix) – November 2013 – 2m,4m,6m,15m,4-6y – 80
- DTaP-IPV (Kinrix) – November 2013 – 2m,4m,6m-18m,4-6y – 80
- DTaP-HepB-IPV (Pediarix) – November 2013 – 2m,4m,6m-18m,4-6y – 80
- DTaP-IPV/Hib (Pentacel) – October 2013 – 2m,4m,6m-18m,4-6y – 80
- Hep A (Havrix) – December 2013 – 12m-1y – 20
- Hep A/Hep B (Twinrix) – August 2012 – 12m-1y – 20
- HPV (Gardasil) 3 doses – June 2014 – 11y-12y – 80
- HPV (Gardasil 9) 3 doses – December 2014 – December 2014 – 80
- Influenza (Agriflu) – 2013 – 6m, Annual – 80
- Influenza (Fluarix) – June 2014 – 6m, Annual – 80
- Influenza (Flublok) – March 2014 – 6m, Annual – 20
- Meningococcal (MenB-Trumenba) – October 2015 – 11y,16y – 80
- Pneumococcal (PCV13-Prevnar 13) – January 2014 – 2m,4m,6m,12m – 80
- Rotavirus (RotaTeq) – June 2013 – 2m,4m,6m – 80
- Tdap (Boostrix) – February 2013 – 11y – 80
Conclusions, Consequences, and Final Questions
What I see here with Merck and the development of its Gardasil ® vaccine is a pattern of research, which failed to detect harm or to prove safety. Their studies could have deceived the public and doctors into thinking that their vaccine, Gardasil ®, has passed all safety standards during clinical trials, when in reality, we see that critical protocols were not done.
Based on the information I provided, I have concluded that safety was never proven with this vaccine. Billions of dollars have been made from sales of this vaccine, and many lives have been ruined — some beyond recognition. Even though the number of adverse reactions to Gardasil ®, serious medical injuries, and related cases in vaccine court continue to climb, Merck has kept the long-term interest of its shareholders as its first priority. Sales and profits continue to rise while more and more children are being severely injured every year.
I believe the emotional and mental distress that results from vaccine injury is beyond understanding until you have been there. The families of vaccine injured children spend years going from doctor to doctor, from hospital to hospital, spending everything they have on medications and other procedures, while watching their formerly happy and healthy children fall into chronic illness or have seizures. It just tears out the hearts of parents. It tears mine out just listening to their stories.
I have seen families ripped apart by these vaccines and the injuries they cause. I have seen the lives of young adults and teenagers destroyed by these vaccines. If you look at the numerous medications that are commonly prescribed to treat the symptoms of vaccine injuries, it is clear that the Big Pharma companies such as Merck are continuing to line their pockets with profits — first from the vaccines, and then from the drugs they sell to treat the vaccine injuries.
In my heart I think the FDA, CDC and DHS are co-conspirators. Why? Because they keep pushing to mandate these unsafe injections which cause harm and sterilize individuals, all the while filling the pockets of Merck executives and shareholders.
Merck and all other vaccine manufacturers have liability protection. This industry and the healthcare providers who give these vaccines to patients have immunity under the 1986 National Childhood Vaccine Injury Act. However, there is one major loophole to Big Pharma immunity.
It is possible to sue the manufacturer of a vaccine when the manufacturer is suspected of committing fraud during the process of licensing. If a vaccine has been approved by the Advisory Committee on Immunization Practices (ACIP), and it can be determined that the manufacturer committed fraud in the process of licensing, then legal action could be taken against the manufacturer.
Did Merck commit fraud in the process of licensing Gardasil ®? Were officials at the FDA and CDC aware of the fraud and did they assist Merck despite their legal obligations to protect the public?
What do you think? Did Merck engage in wrongdoing? Do you trust their safety studies for HPV vaccines? What about the other vaccines that Merck sells?
References
[1] Scott S. Field, MD; “New Concerns about the Human Papillomavirus Vaccine,” American College of Pediatricians, January 2016. http://www.acpeds.org/the-college-speaks/position-statements/health-issues/new-concerns-about-the-human-papillomavirus-vaccine
[2] Deirdre Therese Little, MBBS, and Harvey Rodrick Grenville Ward, Bsc(Med), MBChB, DMCOG, FCOG(SA), MMed (O&G); “Adolescent Premature Ovarian Insufficiency Following Human Papillomavirus Vaccination, A Case Series Seen in General Practice,” J Investig Med High Impact Case Rep, 10/28/2014, PMC 4528880. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4528880/
[3] Cynthia Janak; “HPV Vaccine Cocktail Targets not only HPV,” 11/7/2011. http://www.renewamerica.com/columns/janak/111007
[4] “Toxicology and Carcinogens Studies of Polysorbate 80,” (CAS No. 9005-65-6), in F344/N Rats and B6C3F Mice (Feed Studies), U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Toxicology Program, Technical Report Series, No. 415. http://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr415.pdf
[5] Cynthia Janak; “Vaccine mechanism of harm exposed,” 2/10/2015. http://www.renewamerica.com/columns/janak/150210
[6] CDC – Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report (MMWR), Human Papillomavirus Vaccination Coverage Among Adolescents, 2007–2013, and Postlicensure Vaccine Safety Monitoring, 2006–2014 — United States, Weekly, July 25, 2014 / 63(29);620-4. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6329a3.htm#tab1
[7] “Corporate Governance” Merck & Co. Inc., Kenilworth, N. J. USA. http://www.msdresponsibility.com/ethics-transparency/corporate-governance
[8] “Clinical Review of Biologics License Application Supplement for Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant (Gardasil®) to extend indication for prevention of vaginal and vulvar cancers related to HPV types 16 and 18.” September 11, 2008, BL
[9] “Toxicity Effects,” CAS Registry Number: 9005-65-6, Polysorbate 80, National Toxicology Program. http://tools.niehs.nih.gov/cebs3/ntpviews/index.cfm?action=testarticle.toxicity&cas_number=9005-65-6
[10] Goldman GS and Miller NZ; “Relative trends in hospitalizations and mortality among infants by the number of vaccine doses and age, based on the Vaccine Adverse Event Reporting System (VAERS), 1990–2010,” Human and Experimental Toxicology, October 2012, PMID 22531966. http://het.sagepub.com/content/31/10/1012.full
Gardasil: The Decision We Will Always Regret
The Gardasil Vaccine After-Life: My Daughter is a Shadow of Her Former Self
Gardasil: An Experience no Child Should Have to Go Through
I Want my Daughter’s Life Back the Way it was Before Gardasil
Gardasil Vaccine: Destroyed and Abandoned
15-Year-Old Vaccinated by Force with Gardasil now Suffers from Paralysis and Pain
Recovering from my Gardasil Vaccine Nightmare
Gardasil: We Thought It Was The Right Choice
“HPV Vaccine Has Done This to My Child”
13 Year Old World Championship Karate Student Forced to Quit After Gardasil Vaccine
If I Could Turn Back Time, Korey Would not Have Received any Gardasil Shots
What Doctors Don’t Tell You: Our Gardasil Horror Story
Family Fights U.S. Government over Compensation for Gardasil Vaccine Injuries
Gardasil: When Will our Nightmare End?
HPV Vaccine Injuries: “I Cannot Begin to Describe What it is Like to Watch your Daughter Live in Such Agony”
Gardasil: Don’t Let Your Child Become “One Less”
The Gardasil Vaccine Changed Our Definition of “Normal”
Gardasil: I Should Have Researched First
“They’ve Been Robbed of Their Womanhood” – Local Milwaukee Media Covers Gardasil Vaccine Injuries
Gardasil: The Day Our Daughter’s Life Changed
Gardasil: The Decision I will Always Regret
Gardasil Vaccine: One More Girl Dead
Gardasil: A Parent’s Worst Nightmare
After Gardasil: I Simply Want my Healthy Daughter Back
Gardasil: My Family Suffers with Me
Gardasil Changed my Health, my Life, and Family’s Lives Forever
Gardasil: Ashlie’s Near-Death Experience
Gardasil: My Daughter’s Worst Nightmare
My Personal Battle After the Gardasil Vaccine
Gardasil: The Worst Thing That Ever Happened to Me
A Ruined Life from Gardasil
HPV Vaccines: My Journey Through Gardasil Injuries
The Dark Side of Gardasil – A Nightmare that Became Real
Toddler Wrongly Injected with Gardasil Vaccine Develops Rare Form of Leukaemia
More information about Gardasil
Medical Doctors Opposed to Forced Vaccinations – Should Their Views be Silenced?
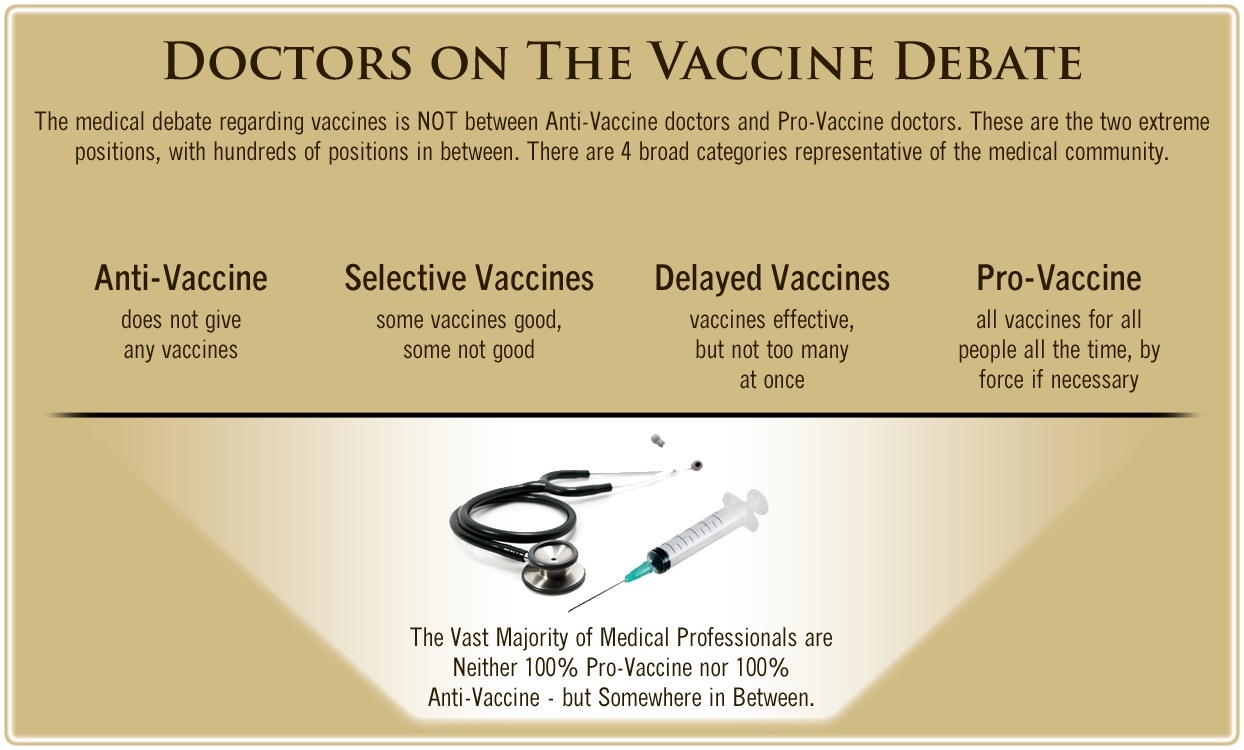
One of the biggest myths being propagated in the compliant mainstream media today is that doctors are either pro-vaccine or anti-vaccine, and that the anti-vaccine doctors are all “quacks.”
However, nothing could be further from the truth in the vaccine debate. Doctors are not unified at all on their positions regarding “the science” of vaccines, nor are they unified in the position of removing informed consent to a medical procedure like vaccines.
The two most extreme positions are those doctors who are 100% against vaccines and do not administer them at all, and those doctors that believe that ALL vaccines are safe and effective for ALL people, ALL the time, by force if necessary.
Very few doctors fall into either of these two extremist positions, and yet it is the extreme pro-vaccine position that is presented by the U.S. Government and mainstream media as being the dominant position of the medical field.
In between these two extreme views, however, is where the vast majority of doctors practicing today would probably categorize their position. Many doctors who consider themselves “pro-vaccine,” for example, do not believe that every single vaccine is appropriate for every single individual.
Many doctors recommend a “delayed” vaccine schedule for some patients, and not always the recommended one-size-fits-all CDC childhood schedule. Other doctors choose to recommend vaccines based on the actual science and merit of each vaccine, recommending some, while determining that others are not worth the risk for children, such as the suspect seasonal flu shot.
These doctors who do not hold extreme positions would be opposed to government-mandated vaccinations and the removal of all parental exemptions.
In this article, I am going to summarize the many doctors today who do not take the most extremist pro-vaccine position, which is probably not held by very many doctors at all, in spite of what the pharmaceutical industry, the federal government, and the mainstream media would like the public to believe.






2 Comments