CENSORSHIP: CDC Takes Over Frontline Doctors’ Website and Replaces Content with Their Own Data
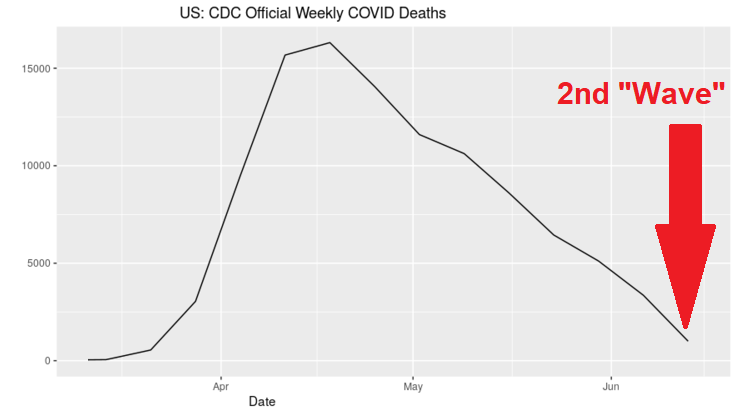
As we have been reporting this week, a group of doctors who have been on the front lines treating COVID patients, successfully, descended upon Washington D.C. this week to conduct press conferences and a 2-day "White Coat Summit" to share their experiences in treating, and curing, their COVID patients. They claim that they represent "thousands" of doctors who have been censored. Their first press conference was sparsely attended by the Washington D.C. media, and the only media company that filmed it and shared it online, Breitbart News, was immediately censored, and the video was quickly deleted from Facebook, YouTube, and Twitter. But the video of that press conference has been preserved, and has now been viewed by over 20 million people. The next day, the Frontline Doctors' website, which used to be at www.americasfrontlinedoctors.com, was removed by the company that was hosting it. Two days ago, the same day as the first press conference, someone bought the domain americasfrontlinedoctors.org, which now displays the CDC's official website about COVID-19. The reason why the U.S. Government and their "health" agencies, as well as Big Tech, are censoring this information is very simple: cures to diseases are not profitable. Millions of Americans are out of work, tens of thousands of small businesses have closed, and the largest transfer of wealth in the history of the United States has occurred during the past few months, allocating close to 2 TRILLION dollars to Big Pharma, most of it for COVID vaccines. And all of this is a CRIMINAL ACT against the American people, if what these Frontline Doctors say is true, which is that there is a simple cure for COVID, and that "nobody has to die" from it. When you understand what is truly happening in America and around the world today, then it is very easy to understand why Big Pharma, Big Tech, and the U.S. Government, all of whom will profit from COVID vaccines and interventions, while at the same time putting into place massive surveillance systems to take away our freedoms, would want to silence this group of doctors who simply want to stop their patients from dying due to the COVID fear. Let's start calling this what it really is. This is MASS MURDER, with crimes against humanity being committed which should be prosecuted as TREASON.