
by Christina England
Health Impact News
When a woman becomes pregnant, naturally, she would want to protect her unborn child above all else. Therefore, when offered a series of vaccinations said to protect her newborn baby against disease in the first few weeks of life, she will probably accept the vaccinations without a moment’s hesitation.
However, would she accept those vaccinations so readily if she knew that her unborn child was going to be used as part of a vaccine experiment being conducted by the Centers for Disease Control and Prevention (CDC) and the vaccine manufacturers?
The Growing Fetus Marked as Big Pharma’s Latest Guinea Pig
According to CDC paperwork, both the Tdap and the Dtap are vaccinations offered to pregnant women during pregnancy, supposedly to protect their newborn infant from contracting pertussis (whooping cough) in the first few weeks of life.
However, despite recommending these vaccinations to all pregnant women, the CDC readily admits in their own documentation that neither vaccine has ever been tested during pregnancy for vaccine safety and that they have no idea whether the vaccines could harm a growing fetus.
In other words, by recommending these vaccinations to pregnant women, the CDC is fully prepared to use unborn babies as part of a massive vaccine experiment. What is even more worrying is the fact that, in doing this, they are potentially risking the lives of millions of unborn babies.
Why would the CDC do this?
Health Impact News decided to investigate this issue more carefully, and what we uncovered may shock and horrify you.
What a Difference Three Years Can Make
In 2008, as recommended by the Advisory Committee on Immunization Practices (ACIP) the CDC stated the following, at the beginning of the MMWR report, titled Prevention of Pertussis, Tetanus, and Diphtheria Among Pregnant and Postpartum Women and Their Infants – Recommendations of the Advisory Committee on Immunization Practices (ACIP):
“Available evidence does not address the safety of Tdap for pregnant women, their fetuses, or pregnancy outcomes sufficiently. Available data also do not indicate whether Tdap-induced transplacental maternal antibodies provide early protection against pertussis to infants or interfere with an infant’s immune responses to routinely administered pediatric vaccines.”
These few sentences alone indicate that this vaccine is clearly unsafe for pregnant women and that there is no data to suggest that this vaccine can offer early protection. The CDC further supports this perspective by providing the following information in the introduction of their report:
“The safety and efficacy of using Tdap in pregnant women has not been demonstrated, and Tdap is not recommended for use in pregnant women in any country. No evidence exists of excess morbidity or any fatality among pregnant women ascribed to pertussis. No evidence exists demonstrating whether
-
Tdap in pregnant women harms the fetus or increases risk for adverse pregnancy outcomes,
-
transplacental antibody induced by Tdap administered during pregnancy will protect infants against pertussis, or
-
Tdap-induced transplacental maternal antibody will have a negative impact on an infant’s protective immune response to later-administered routine pediatric DTaP or to conjugate vaccines containing tetanus toxoid or diphtheria toxoid.”
With this information in mind, we need to ask ourselves how, three years later, the Tdap, an untested, non-recommended vaccine can suddenly be recommended as not only safe but necessary by the CDC and offered to all pregnant women to protect their newborn baby from whooping cough.
This is especially worrying when you consider the fact that the vaccine manufacturers themselves cannot provide any evidence to suggest that this vaccine is either safe or effective.
Manufacturers Admit Vaccinations Untested for Safety in Pregnancy
First, let us study the vaccine information sheets for the two Tdap vaccinations that the CDC recommends for pregnant women, Adacel and Boostrix, both manufactured to protect against tetanus, diphtheria and pertussis (whooping cough).
Adacel, a Vaccine Manufactured by Sanofi Pasteur
In section 8 of the vaccines information leaflet titled Use in Specific Populations, Sanofi Pasteur stated:
“8.1
Pregnancy Category C
Animal reproduction studies have not been conducted with Adacel vaccine. It is also not known whether Adacel vaccine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Adacel vaccine should be given to a pregnant woman only if clearly needed.” (own emphasis)
In their information sheet, Sanofi Pasteur did reference the fact that they had carried out limited testing on pregnant animals; however, they made it abundantly clear that Adacel had never been tested for vaccine safety in pregnant women and therefore they do not know if the vaccine could harm a human fetus.
Interestingly, the manufacturer stated that the Adacel vaccine should only be given to a pregnant mother if it is “clearly needed,” however, what they do not say is what constitutes being “clearly needed.”
How can any vaccine, EVER, be “clearly needed” for use with pregnant women?
Next, we will look at the information sheet for Boostrix, manufactured by GlaxoSmithKline to once again protect us against tetanus, diphtheria and pertussis.
Boostrix, a Vaccine Manufactured by GlaxoSmithKline
Once again, in section 8, titled Use in Specific Populations, the manufacturer has stated:
“8.1 Pregnancy
Pregnancy Category B
A developmental toxicity study has been performed in female rats at a dose approximately 40 times the human dose (on a mL/kg basis) and revealed no evidence of harm to the fetus due to BOOSTRIX. Animal fertility studies have not been conducted with BOOSTRIX. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, BOOSTRIX should be given to a pregnant woman only if clearly needed.” (own emphasis)
Once again, the manufacturer of Boostrix also mentioned that the vaccine had been tested on pregnant animals. However, GlaxoSmithKline had stated that no adequate or well-controlled studies had been carried out on pregnant women. In other words, they also had no idea if their vaccine would be safe during pregnancy or could cause harm to the growing fetus.
GlaxoSmithKline also stated that this vaccine should only be given to a pregnant woman if it is clearly needed.
CDC Willing to Put Unborn Babies at Risk
Despite having no proof that these vaccinations are safe to give pregnant women, in 2011, the CDC decided to do a complete turnaround. They decided to go ahead and recommend the vaccination anyway.
Is the CDC an organization that cares if these vaccinations have been found to be safe for use in pregnant women? As we have previously reported here at Health Impact News, the CDC is the largest vendor purchasing vaccines, spending over $4 billion annually to purchase vaccines, which is a serious conflict of interest in overseeing vaccine safety. (See: CDC’s Purchase of $4 Billion of Vaccines a Conflict of Interest in Overseeing Vaccine Safety.)
The Year the CDC Recommended Untested Vaccines in Pregnancy
In 2011, the CDC began their updated recommendation paper by explaining that the majority of children who are hospitalized and who die from whooping cough are below the age of two months and are therefore too young to be vaccinated. It is for this reason that they feel it is necessary to put another strategy in place.
To reassure the public that all is well and that they have checked for vaccine safety, the CDC explained that although they are fully aware that the vaccines have not been tested for safety in pregnancy, they have used the pregnancy registries put in place by the vaccine manufacturers and small studies to collect up-to-date safety data.
Again, using the advice given to them by the AICP, the CDC stated:
“…, ACIP made recommendations for use of Tdap in unvaccinated pregnant women and updated recommendations on cocooning and special situations.”
By making this statement, the CDC has indicated that they agreed with the ACIP and recommended that the Tdap should be offered to pregnant women, for the protection of their unborn babies in the first few weeks of their lives.
The CDC continued by stating that:
“In pre-licensure evaluations, the safety of administering a booster dose of Tdap to pregnant women was not studied.”
However, they then attempted to reassure pregnant women by stating that:
“Because information on use of Tdap in pregnant women was lacking, both manufacturers of Tdap established pregnancy registries to collect information and pregnancy outcomes from pregnant women vaccinated with Tdap. Data on the safety of administering Tdap to pregnant women are now available. ACIP reviewed published and unpublished data from VAERS, Sanofi Pasteur (Adacel) and GlaxoSmithKline (Boostrix) pregnancy registries, and small studies. ACIP concluded that available data from these studies did not suggest any elevated frequency or unusual patterns of adverse events in pregnant women who received Tdap and that the few serious adverse events reported were unlikely to have been caused by the vaccine.” (own emphasis)
Is the public being warned by their physicians regarding the risks and unknown safety of this vaccine being administered to pregnant women?
What Happened to Vaccine Protocol?
Before any vaccine is marketed as safe and effective, it has to go through a series of clinical trials. These trials are usually carried out in three phrases. Phase 1 includes safety and immunogenicity studies performed in a small number of closely monitored subjects. Phase 2 studies are dose-ranging studies and may enroll hundreds of subjects. Finally, phase 3 trials typically enroll thousands of individuals and provide the critical documentation of effectiveness and important additional safety data required for licensing.
This procedure is usually put in place by the FDA.
If a vaccine is going to be recommended for use on a particular group of individuals, you would have expected the manufacturers to have carried out extensive testing beforehand, especially on vulnerable individuals like pregnant women and their unborn babies.
The vaccine adverse event reporting system (VAERS) and the manufacture’s registries are incapable of providing scientific evidence of vaccine safety. This is because they have no control group (and obviously are non-randomized or blinded) and therefore their data has no validity in establishing a safety record for using the vaccine in pregnant women.
We need to ask ourselves, how a vaccine can be recommended for use in pregnancy if no clinical trials have been carried out on pregnant women.
Two Studies Provided Insignificant Evidence to Support Vaccine Safety
When you read the CDC 2011 paper carefully, you will notice that they did reference two small studies that they have used to provide evidence of the vaccines’ safety in pregnancy. However, we believe that the numbers of participants were too small to provide substance for their claims.
The first study referenced by the CDC was a study titled Maternal immunization with tetanus–diphtheria–pertussis vaccine: effect on maternal and neonatal serum antibody levels, carried out by Stanley A. Gall, MD; John Myers, PhD; and Michael Pichichero, MD.
The researchers studied a total of 104 pregnant women and vaccinated 52 of them.
Although the researchers stated that there were no adverse reactions, no safety data was provided; therefore, we only have their word for it.
The researchers concluded:
“Administering Tdap during pregnancy increases antibody titers against diphtheria and pertussis antigens. Maternal Tdap may prevent neonatal pertussis infection.” (own emphasis)
Considering these vaccines are advised for pregnant women to protect newborn infants from contracting pertussis during the first eight weeks of life, this study did not provide any substantial evidence to support the safety or effectiveness of either vaccine. Nor did the study present us with any evidence to support whether or not the vaccine could actually protect newborn infants against the pertussis infection.
This is especially worrying, as the researchers have stated that the objective of this study was to determine whether or not offering the Tdap in pregnancy protected newborn infants against pertussis:
“We sought to determine whether tetanus– diphtheria– pertussis vaccination (Tdap) in pregnancy provides newborns antibodies against pertussis when compared to mothers who did not receive Tdap.”
It is also worth noting that the lead researcher, Stanley A. Gall, M.D., had received grants and research support from two leading vaccine manufacturers, GlaxoSmithKline, the manufacturer of Boostrix, and Merck, where he served as a consultant.
The website Protect cme.org states that:
“Dr. Stanley Gall has received grants/research support from GlaxoSmithKline and Merck, has served as a consultant for Merck, and has received honoraria from GlaxoSmithKline and Merck.”
Since the main researcher had conflicts of interest, how can this study be used as evidence?
The second of two studies referenced was conducted by Elizabeth A. Talbot, Kristin H. Brown, Kathryn B. Kirkland, Andrew L. Baughman, Scott A. Halperin, and Karen R. Broder, titled The safety of immunizing with tetanus–diphtheria–acellular pertussis vaccine (Tdap) less than 2 years following previous tetanus vaccination: Experience during a mass vaccination campaign of healthcare personnel during a respirator illness outbreak.
Once again, this study proved less than convincing, as the researchers only vaccinated a total of 16 pregnant women with the Tdap vaccination, and although various adverse events were reported throughout the study, these were shrugged off by the CDC in their report as being unrelated to the vaccine.
Conclusion: CDC Cannot Be Trusted for Vaccine Safety of Unborn Children
It is clear from our investigations that the CDC has decided to recommend a potentially unsafe and ineffective vaccination for use in pregnancy, even though they cannot provide any convincing data to reassure mothers that it cannot harm their growing fetus.
Both studies used as evidence by the CDC only tested a small sample group, providing inadequate data to prove vaccine safety. Any adverse events that were reported by the researchers were shrugged off by the CDC and said to be unlikely to have been caused by the vaccine.
As the CDC did not present any longitudinal studies to show whether or not this vaccine can cause any long term harm to children, how can parents be confident that it is safe?
Acknowledgement: The assistance of a distinguished Israeli vaccine researcher who prefers not to be named was greatly appreciated.
Medical Doctors Opposed to Forced Vaccinations – Should Their Views be Silenced?
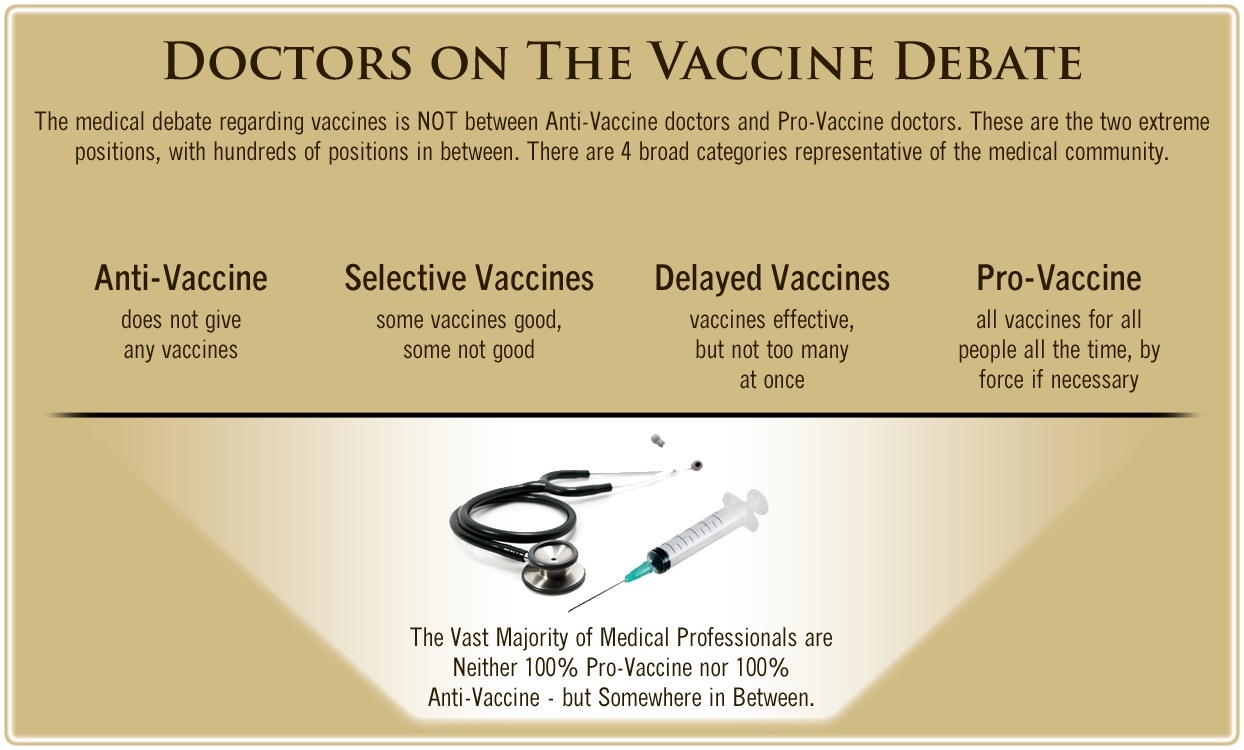
One of the biggest myths being propagated in the compliant mainstream media today is that doctors are either pro-vaccine or anti-vaccine, and that the anti-vaccine doctors are all “quacks.”
However, nothing could be further from the truth in the vaccine debate. Doctors are not unified at all on their positions regarding “the science” of vaccines, nor are they unified in the position of removing informed consent to a medical procedure like vaccines.
The two most extreme positions are those doctors who are 100% against vaccines and do not administer them at all, and those doctors that believe that ALL vaccines are safe and effective for ALL people, ALL the time, by force if necessary.
Very few doctors fall into either of these two extremist positions, and yet it is the extreme pro-vaccine position that is presented by the U.S. Government and mainstream media as being the dominant position of the medical field.
In between these two extreme views, however, is where the vast majority of doctors practicing today would probably categorize their position. Many doctors who consider themselves “pro-vaccine,” for example, do not believe that every single vaccine is appropriate for every single individual.
Many doctors recommend a “delayed” vaccine schedule for some patients, and not always the recommended one-size-fits-all CDC childhood schedule. Other doctors choose to recommend vaccines based on the actual science and merit of each vaccine, recommending some, while determining that others are not worth the risk for children, such as the suspect seasonal flu shot.
These doctors who do not hold extreme positions would be opposed to government-mandated vaccinations and the removal of all parental exemptions.
In this article, I am going to summarize the many doctors today who do not take the most extremist pro-vaccine position, which is probably not held by very many doctors at all, in spite of what the pharmaceutical industry, the federal government, and the mainstream media would like the public to believe.





3 Comments