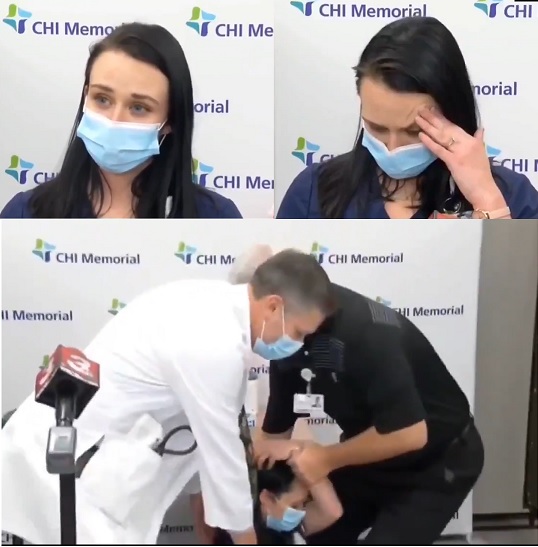
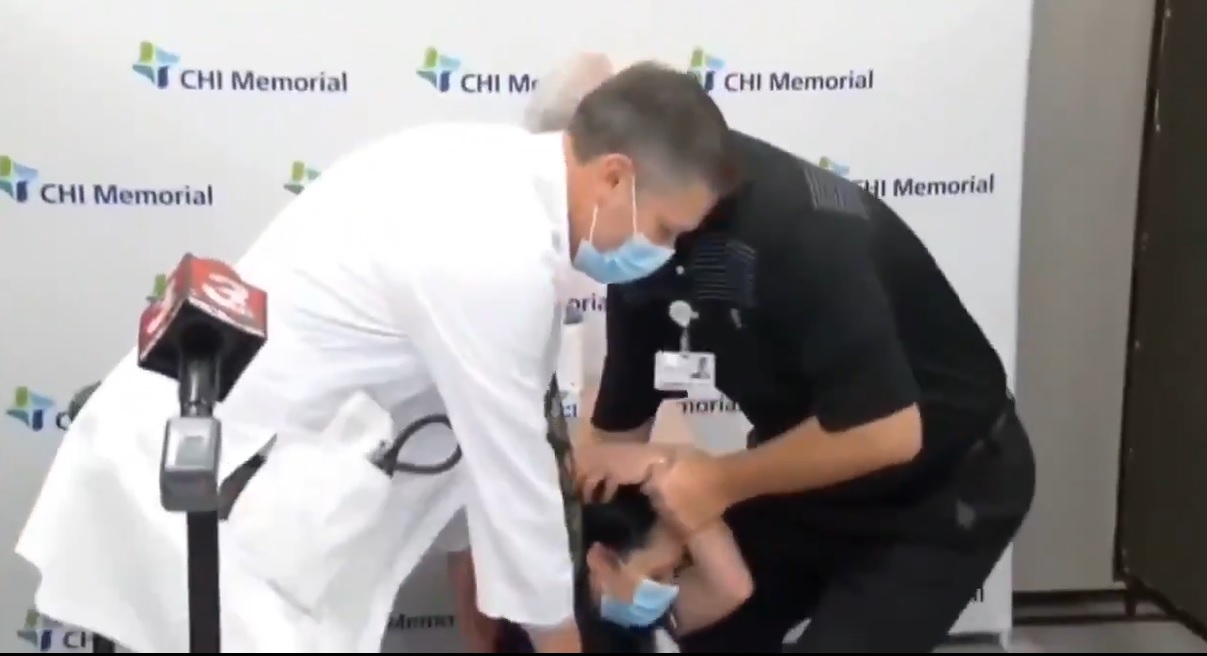
WARNING: 3,150 Injuries in First Week of Illegal Experimental COVID Vaccines Among American Healthcare Workers! Pregnant Women Included
The first week of injecting American healthcare workers with the experimental illegal Pfizer mRNA vaccine has resulted in over 3000 of these healthcare workers reporting that they were injured to the extent that they could not continue on their jobs and perform normal activities, requiring care from a doctor or healthcare worker. This report is directly from the CDC and was published yesterday, December 19, 2020. If the staffing issues and overcrowding at hospitals across the U.S. were being over-exaggerated in the Pharma-controlled corporate media in recent weeks to instill fear over COVID to the public, that is all about to change as the next phase of the experimental COVID vaccine trials is being conducted on the American public, starting with healthcare workers this past week. Because according to this CDC report, the healthcare system just lost over 3000 staff due to the experimental COVID vaccine, and not COVID itself. Using healthcare workers as the first human lab rats in the public was obviously planned from the start. Those trained in the dogma of the pharmaceutical industry, at least many of them, were able to roll up their sleeves and get this experimental vaccine and be convinced that the side effects are "normal" and for the "greater good," because that is what their training has taught them. This will also lead to REAL hospital over-crowding as more and more of these healthcare workers will not be able to show up for work next week, and probably the weeks ahead, and the pharma-controlled corporate media will spin this as being due to the rising "cases" of COVID to convince the general public to line up and get this vaccine. Next up will be seniors in assisted living institutions, probably beginning this week, and most of them have co-morbidity conditions with weakened immune systems and are pumped full of toxic pharmaceutical drugs. This will be a literal holocaust, and be far worse than the tens of thousands of seniors who died when COVID started! The other disturbing aspect of the CDC report published yesterday is that there were 514 healthcare workers pregnant that received the first round of Pfizer's experimental vaccine. WAKE UP AMERICA!!