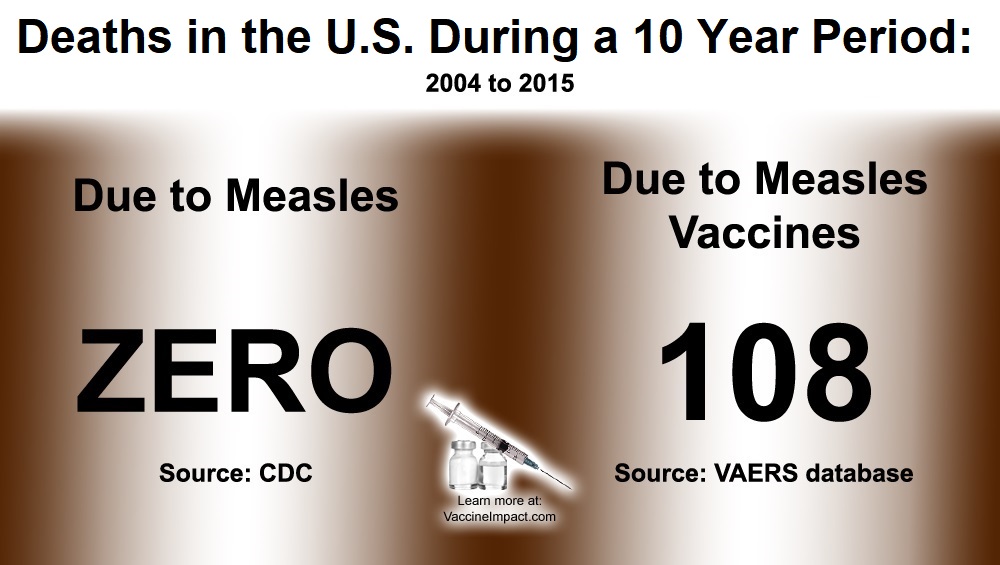
Victory for Toronto Nurses: Rationale for Nurses Who Refuse Flu Vaccine to Wear Masks “Insufficient, Inadequate, and Completely Unpersuasive”
The Ontario Nurses' Association has won a major victory over St. Michael's Hospital and eight other Toronto-area hospitals, as labour arbitrator William Kaplan wrote in a 53-page decision that forcing unvaccinated nurses to wear surgical masks during the flu season is a policy that is "insufficient, inadequate, and completely unpersuasive.” The hospital failed to prove that nurses who didn't have flu symptoms were a significant source of infection and therefore would be required to wear masks if they weren't inoculated, Mr. Kaplan said in his decision. Mr. Kaplan also heard an expert, Lisa Brosseau, a professor of environmental and occupational health sciences, testify that surgical masks fit poorly and aren't an effective form of protection. The arbitrator said it was illogical to force nurses who aren't immunized to be masked when St. Michael’s isn't as strict with unvaccinated visitors. "If masking is truly effective as source control, how can it be that they too are not required to mask? The answer to this question reveals that the masking part of the policy is, as one St. Michael’s witness admitted, 'weak,' ” Mr. Kaplan wrote.